Difference between revisions of "Subliming"
(Created page with "==Key Stage 3== ===Meaning=== '''Subliming''' is when a liquid turns into a gas. : Noun: '''Sublimation''' : Verb: '''To sublime''' : Present Participle:...") |
|||
| Line 8: | Line 8: | ||
===About Subliming=== | ===About Subliming=== | ||
| − | : Only a few [[solid]]s '''sublime'''. | + | : Only a few [[solid]]s '''sublime'''. [[Freezing|Frozen]] [[Carbon Dioxide]] will [[sublime]] which means it skips the [[liquid]] [[State of Matter|state]]. |
: '''Sublimation''' is a [[Reversible Changes|reversible]] process. When a [[solid]] '''sublimes''' into a [[gas]] you can [[depositing|deposit]] that [[gas]] back into a [[solid]]. | : '''Sublimation''' is a [[Reversible Changes|reversible]] process. When a [[solid]] '''sublimes''' into a [[gas]] you can [[depositing|deposit]] that [[gas]] back into a [[solid]]. | ||
: Heating a [[solid]] will cause it to '''sublime''' into a [[gas]]. | : Heating a [[solid]] will cause it to '''sublime''' into a [[gas]]. | ||
Revision as of 16:01, 22 September 2018
Key Stage 3
Meaning
Subliming is when a liquid turns into a gas.
- Noun: Sublimation
- Verb: To sublime
- Present Participle: Subliming
About Subliming
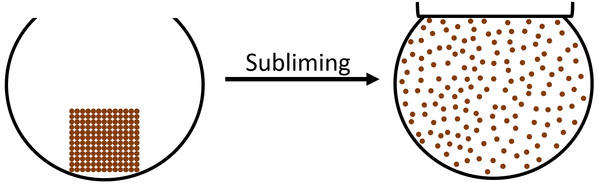
- Only a few solids sublime. Frozen Carbon Dioxide will sublime which means it skips the liquid state.
- Sublimation is a reversible process. When a solid sublimes into a gas you can deposit that gas back into a solid.
- Heating a solid will cause it to sublime into a gas.
| The particles in the solid vibrate faster until they are moving fast enough that they break the bonds holding the particles together. The particles become free to move anywhere which makes the state a gas. |