Difference between revisions of "Subliming"
| Line 19: | Line 19: | ||
| style="height:20px; width:600px; text-align:left;" |The [[particle]]s in the [[solid]] [[vibrate]] faster until they are moving fast enough that they break the [[bond]]s holding the [[particle]]s together. The [[particle]]s become free to move anywhere which makes the [[State of Matter|state]] a [[gas]]. | | style="height:20px; width:600px; text-align:left;" |The [[particle]]s in the [[solid]] [[vibrate]] faster until they are moving fast enough that they break the [[bond]]s holding the [[particle]]s together. The [[particle]]s become free to move anywhere which makes the [[State of Matter|state]] a [[gas]]. | ||
|} | |} | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | [[Subliming]] is an [[endothermic]] [[Physical Change|physical change]] in which a [[solid]] turns into a [[gas]] without going through a [[liquid]] [[State of Matter|phase]]. | ||
| + | |||
| + | ===About Subliming=== | ||
| + | : [[Subliming]] happens when the [[particle]]s in a [[solid]] break [[bond]]s holding them together in fixed positions as they gain [[Potential Energy|potential energy]]. | ||
| + | : [[Subliming]] is an [[endothermic]] process, which means it needs to [[Absorb (Physics)|absorb]] [[energy]] to take place. | ||
| + | : [[Subliming]] is a [[Physical Change|physical change]], which means it is [[Reversible Changes|reversible]] and does not produce new [[chemical]]s. | ||
| + | : [[Substance]]s may [[sublime]] depending on the [[pressure]] around them. In [[Atmospheric Pressure|atmospheric pressure]] (101,000[[Pa]]) [[Carbon Dioxide]] and [[Iodine]] will both [[sublime]] and cannot exist in a [[liquid]] [[State of Matter|state]]. However, under extremely high [[pressure]] [[Carbon Dioxide]] can be turned into a [[liquid]]. | ||
| + | : [[Ice]] will [[sublime]] in a [[vacuum]] but not in normal [[Atmospheric Pressure|atmospheric pressure]]. | ||
Revision as of 11:57, 27 December 2018
Contents
Key Stage 3
Meaning
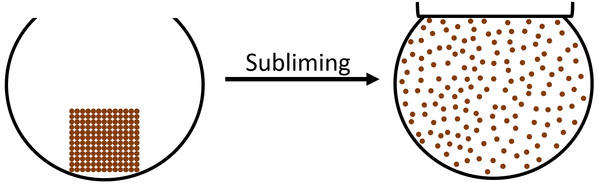
Subliming is an endothermic process in which a solid turns directly into a gas without going through a liquid state.
- Noun: Sublimation
- Verb: To sublime
- Present Participle: Subliming
About Subliming
- Only a few solids sublime. Frozen Carbon Dioxide will sublime which means it skips the liquid state.
- Sublimation is a reversible process. When a solid sublimes into a gas you can deposit that gas back into a solid.
- Heating a solid will cause it to sublime into a gas.
| The particles in the solid vibrate faster until they are moving fast enough that they break the bonds holding the particles together. The particles become free to move anywhere which makes the state a gas. |
Key Stage 4
Meaning
Subliming is an endothermic physical change in which a solid turns into a gas without going through a liquid phase.
About Subliming
- Subliming happens when the particles in a solid break bonds holding them together in fixed positions as they gain potential energy.
- Subliming is an endothermic process, which means it needs to absorb energy to take place.
- Subliming is a physical change, which means it is reversible and does not produce new chemicals.
- Substances may sublime depending on the pressure around them. In atmospheric pressure (101,000Pa) Carbon Dioxide and Iodine will both sublime and cannot exist in a liquid state. However, under extremely high pressure Carbon Dioxide can be turned into a liquid.
- Ice will sublime in a vacuum but not in normal atmospheric pressure.