Difference between revisions of "Positive Ion"
| Line 5: | Line 5: | ||
===About Positive Ions=== | ===About Positive Ions=== | ||
: In [[Chemical Reaction|chemical reactions]] between [[metal]]s and [[non-metal]]s the [[metal]] [[element]]s form '''positive ions'''. | : In [[Chemical Reaction|chemical reactions]] between [[metal]]s and [[non-metal]]s the [[metal]] [[element]]s form '''positive ions'''. | ||
| − | : [[Hydrogen]] forms '''positive ions''' in some [[compound]]s and it is these [[Hydrogen Ion|H<sup>+</sup> ions]] which can make [[solution]]s [[acid]]ic. | + | : [[Hydrogen]] forms '''positive ions''' in some [[compound]]s and it is these [[Hydrogen Ion (Chemistry)|H<sup>+</sup> ions]] which can make [[solution]]s [[acid]]ic. |
: '''Positive ions''' are attracted to [[Negative Ion|negative ions]] and to the [[Cathode|negative electrode (cathode)]] during [[electrolysis]]. | : '''Positive ions''' are attracted to [[Negative Ion|negative ions]] and to the [[Cathode|negative electrode (cathode)]] during [[electrolysis]]. | ||
Revision as of 17:41, 7 April 2019
Key Stage 4
Meaning
Positive ions are elements which have lost one or more electrons to become positively charged.
About Positive Ions
- In chemical reactions between metals and non-metals the metal elements form positive ions.
- Hydrogen forms positive ions in some compounds and it is these H+ ions which can make solutions acidic.
- Positive ions are attracted to negative ions and to the negative electrode (cathode) during electrolysis.
Examples
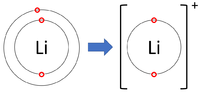
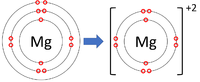
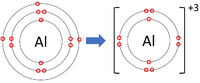
| Lithium forms +1 ions. | Magnesium forms +2 ions. | Aluminium forms +3 ions. |