Difference between revisions of "Group 2"
(Created page with "==Key Stage 3== ===Meaning=== Group 2 are elements on the Periodic Table with only 2 electrons in their outer shell. ===About Group 2=== : G...") |
|||
| (4 intermediate revisions by one other user not shown) | |||
| Line 5: | Line 5: | ||
===About Group 2=== | ===About Group 2=== | ||
: [[Group 2]] [[element]]s are known as the '''Alkali Earth Metals'''. | : [[Group 2]] [[element]]s are known as the '''Alkali Earth Metals'''. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
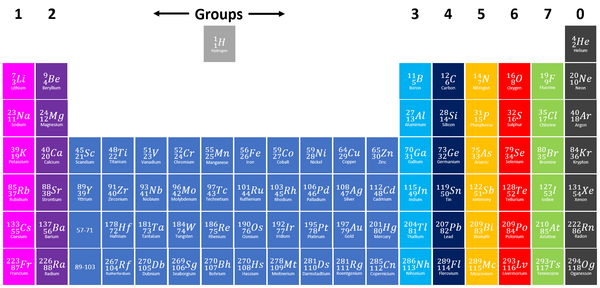
| + | |[[File:PeriodicTableGroups.png|center|600px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Group 2]] [[element]]s are shown in dark purple at the left of the [[Periodic Table]]. | ||
| + | |} | ||
: The [[element]]s in [[Group 2]] are: | : The [[element]]s in [[Group 2]] are: | ||
:*[[Beryllium]] | :*[[Beryllium]] | ||
| Line 18: | Line 24: | ||
===About Group 2=== | ===About Group 2=== | ||
| + | : [[Group 2]] [[atom]]s can lose 2 [[electron]]s to form [[Positive Ion|positive ions]] with a [[Electrical Charge|charge]] of +2. | ||
: [[Group 2]] [[element]]s are known as the '''Alkali Earth Metals'''. | : [[Group 2]] [[element]]s are known as the '''Alkali Earth Metals'''. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:PeriodicTableGroups.png|center|600px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Group 2]] [[element]]s are shown in dark purple at the left of the [[Periodic Table]]. | ||
| + | |} | ||
: The [[element]]s in [[Group 2]] are: | : The [[element]]s in [[Group 2]] are: | ||
:*[[Beryllium]] | :*[[Beryllium]] | ||
| Line 26: | Line 39: | ||
:*[[Barium]] | :*[[Barium]] | ||
:*[[Radium]] | :*[[Radium]] | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Alkali earth metals, page 133, GCSE Chemistry; Student Book, Collins, AQA''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Alkali earth metals; flame tests, page 274-5, GCSE Chemistry; Student Book, Collins, AQA''] | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Group 2 (alkali earth metals), page 133, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Group 2; flame tests, pages 274-5, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
Latest revision as of 08:00, 6 November 2019
Contents
Key Stage 3
Meaning
Group 2 are elements on the Periodic Table with only 2 electrons in their outer shell.
About Group 2
| Group 2 elements are shown in dark purple at the left of the Periodic Table. |
Key Stage 4
Meaning
Group 2 are elements on the Periodic Table with only 2 electrons in their outer shell.
About Group 2
- Group 2 atoms can lose 2 electrons to form positive ions with a charge of +2.
- Group 2 elements are known as the Alkali Earth Metals.
| Group 2 elements are shown in dark purple at the left of the Periodic Table. |
References
AQA
- Alkali earth metals, page 133, GCSE Chemistry; Student Book, Collins, AQA
- Alkali earth metals; flame tests, page 274-5, GCSE Chemistry; Student Book, Collins, AQA