Difference between revisions of "Thermal Equilibrium"
(Created page with "==Key Stage 3== ===Meaning=== Thermal Equilibrium is when all objects in a system are at the same temperature. ===About Thermal Equilibrium==== : When a hot [...") |
|||
| (2 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
[[Thermal Equilibrium]] is when all [[object]]s in a [[system]] are at the same [[temperature]]. | [[Thermal Equilibrium]] is when all [[object]]s in a [[system]] are at the same [[temperature]]. | ||
| − | ===About Thermal Equilibrium | + | ===About Thermal Equilibrium=== |
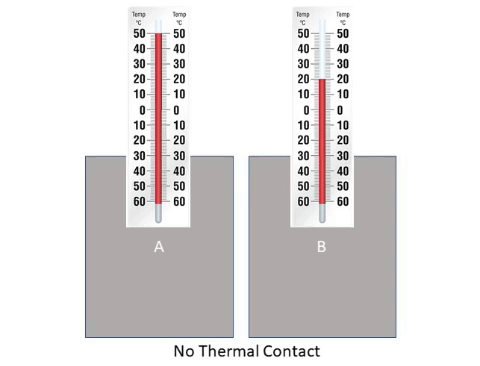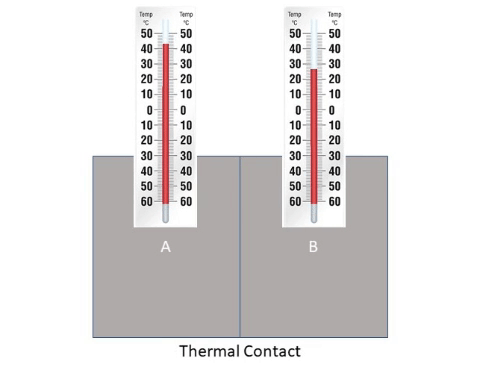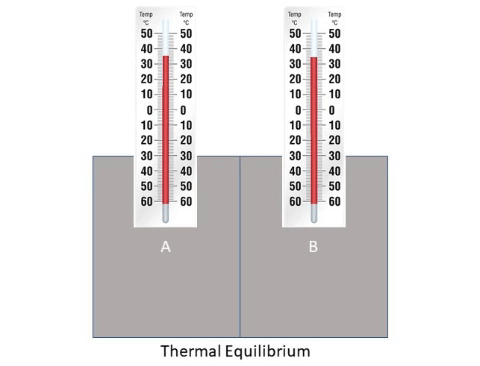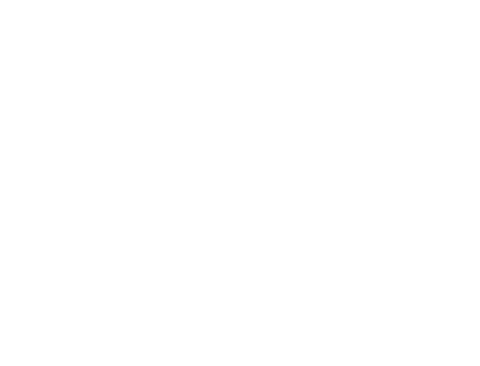
: When a hot [[object]] is placed next to a cold [[object]] [[energy]] will flow from the hotter to the cooler until they reach '''thermal equilibrium'''. | : When a hot [[object]] is placed next to a cold [[object]] [[energy]] will flow from the hotter to the cooler until they reach '''thermal equilibrium'''. | ||
| − | : | + | |
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:ThermalEquilibrium.gif|center|]] | ||
| + | |} | ||
Latest revision as of 14:09, 10 October 2018
Key Stage 3
Meaning
Thermal Equilibrium is when all objects in a system are at the same temperature.
About Thermal Equilibrium
- When a hot object is placed next to a cold object energy will flow from the hotter to the cooler until they reach thermal equilibrium.