Difference between revisions of "Giant Covalent Structure"
(→About Giant Covalent Structures) |
|||
| Line 4: | Line 4: | ||
===About Giant Covalent Structures=== | ===About Giant Covalent Structures=== | ||
| − | : '''Giant covalent structures''' are [[molecule]]s | + | : '''Giant covalent structures''' are [[molecule]]s made of a large number of [[non-metal]] [[atom]]s joined by [[Covalent Bond|covalent bonds]]. |
: The [[atom]]s in a '''giant covalent structure''' are arranged in a regular [[lattice]] (a repeating pattern of [[element]]s. | : The [[atom]]s in a '''giant covalent structure''' are arranged in a regular [[lattice]] (a repeating pattern of [[element]]s. | ||
Revision as of 14:00, 28 December 2018
Contents
Key Stage 4
Meaning
Giant covalent structures are very large molecules in which all the atoms are held to one another by covalent bonds.
About Giant Covalent Structures
- Giant covalent structures are molecules made of a large number of non-metal atoms joined by covalent bonds.
- The atoms in a giant covalent structure are arranged in a regular lattice (a repeating pattern of elements.
Examples
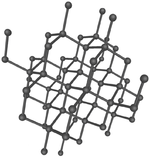
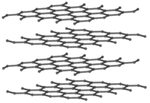
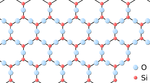
| Diamond is a giant covalent structure where each Carbon atom has 4 bonds with adjacent atoms. | Graphite has a giant covalent structure with each Carbon atom has 3 bonds with adjacent atoms in a layer with loose bonds between the layers. | Graphene has a giant covalent structure with each Carbon atom has 3 bonds with adjacent atoms forming a layer that is one atom thick. | Silica is made of Silicon and Oxygen atoms in a lattice forming a giant covalent structure. |
Bulk Properties
- Giant Covalent Structures usually have very high melting points due to the strong chemical bonds between adjacent atoms.
- Giant Covalent Structures may have good or poor electrical conductors depending on whether there are any electrons free to move between atoms.
- Diamond is a poor electrical conductor because all the electrons are shared by adjacent atoms so none are free to move between atoms.
- Graphite and graphene are good electrical conductors because the Carbon atoms only form 3 bonds with adjacent atoms allowing a spare electron in each atom to move freely around the molecule.