Difference between revisions of "Methanoic Acid"
| Line 31: | Line 31: | ||
[[Methanoic Acid]] can be [[neutralise (Chemistry)|neutralised]] to [[Product|produce]] [[Organic Compound|organic]] [[salt]]s. | [[Methanoic Acid]] can be [[neutralise (Chemistry)|neutralised]] to [[Product|produce]] [[Organic Compound|organic]] [[salt]]s. | ||
: [[Methanoic Acid]] + [[Sodium]] → [[Sodium Methanoate]] + [[Hydrogen]] | : [[Methanoic Acid]] + [[Sodium]] → [[Sodium Methanoate]] + [[Hydrogen]] | ||
| − | : < | + | : <math>2HCOOH + 2Na → 2HCOONa + H_2</math> |
: [[Methanoic Acid]] + [[Potassium Oxide]] → [[Potassium Methanoate]] + [[Water]] | : [[Methanoic Acid]] + [[Potassium Oxide]] → [[Potassium Methanoate]] + [[Water]] | ||
| − | : < | + | : <math>2HCOOH + 2K → 2HCOOK + H_2O</math> |
: [[Methanoic Acid]] + [[Lithium Hydroxide]] → [[Lithium Methanoate]] + [[Water]] | : [[Methanoic Acid]] + [[Lithium Hydroxide]] → [[Lithium Methanoate]] + [[Water]] | ||
| − | : < | + | : <math>HCOOH + LiOH → HCOOLi + H_2O</math> |
: [[Methanoic Acid]] + [[Magnesium Carbonate]] → [[Magnesium Methanoate]] + [[Carbon Dioxide]] + [[Water]] | : [[Methanoic Acid]] + [[Magnesium Carbonate]] → [[Magnesium Methanoate]] + [[Carbon Dioxide]] + [[Water]] | ||
| − | : < | + | : <math>2HCOOH + MgCO_3 → (HCOO)_2Mg + CO_2 + H_2O</math> |
Revision as of 14:22, 7 June 2019
Contents
Key Stage 3
Meaning
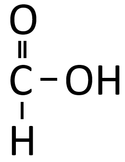
Methanoic Acid is a compound with chemical formula CH2O2.
About Methanoic Acid
- Methanoic Acid is a solid dissolved in water to form a solution.
Methanoic Acid can be neutralised to produce organic salts.
- Methanoic Acid + Sodium → Sodium Methanoate + Hydrogen
- Methanoic Acid + Potassium Oxide → Potassium Methanoate + Water
- Methanoic Acid + Lithium Hydroxide → Lithium Methanoate + Water
- Methanoic Acid + Magnesium Carbonate → Magnesium Methanoate + Carbon Dioxide + Water
Key Stage 4
Meaning
Methanoic Acid is a Carboxylic Acid with chemical formula CH2O2.
About Methanoic Acid
- Methanoic Acid is an aqueous solution.
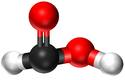
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| CH2O2 | HCOOH |
Methanoic Acid can be neutralised to produce organic salts.
\[2HCOOH + 2Na → 2HCOONa + H_2\]
\[2HCOOH + 2K → 2HCOOK + H_2O\]
\[HCOOH + LiOH → HCOOLi + H_2O\]
\[2HCOOH + MgCO_3 → (HCOO)_2Mg + CO_2 + H_2O\]