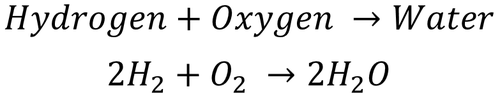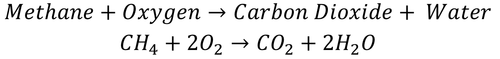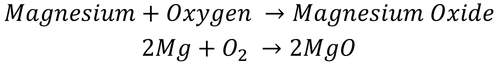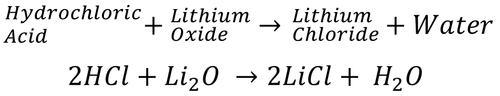Difference between revisions of "Balanced Symbol Equation"
| Line 8: | Line 8: | ||
{| class="wikitable" | {| class="wikitable" | ||
| + | |+ The [[Word Equation]] has been given above each Balanced Symbol Equation. | ||
|- | |- | ||
| − | |[[File:2H2+O2.png|center| | + | |[[File:2H2+O2.png|center|500px]] |
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:500px; text-align:center;" |This shows there are 2 H<sub>2</sub> [[molecule]]s and 1 O<sub>2</sub> [[molecule]] needed to make 2 [[molecules]] of H<sub>2</sub>O. |
|- | |- | ||
| − | |[[File:CH4+2O2.png|center| | + | |[[File:CH4+2O2.png|center|500px]] |
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:500px; text-align:center;" |Text |
|- | |- | ||
| − | |[[File:2Mg+O2.png|center| | + | |[[File:2Mg+O2.png|center|500px]] |
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:500px; text-align:center;" |Text |
|- | |- | ||
| − | |[[File:2HCl+Li2O.png|center| | + | |[[File:2HCl+Li2O.png|center|500px]] |
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:500px; text-align:center;" |Text |
|} | |} | ||
Revision as of 10:02, 28 September 2018
Key Stage 3
Meaning
A Balanced Symbol Equation is a way to show the reactants and products in a chemical reaction using the chemical formulae of the reactants and products.
About Balanced Symbol Equations
- Balanced Symbol Equations shows the number of molecules of each chemical and the numbers of each atom within those chemicals.
- Balanced Symbol Equations can be used to find the quantity of each chemical needed to have a complete chemical reaction.
| This shows there are 2 H2 molecules and 1 O2 molecule needed to make 2 molecules of H2O. |
| Text |
| Text |
| Text |