Contents
Key Stage 2
Meaning
An electrical conductor is a material that allows electricity to flow through it easily.
- Singular Noun: Electrical conductor
- Plural Noun: Electrical conductors
- Verb: To electrically conduct
- Adjective: Electrically conductive
About Electrical Conductors
| Metal is a good electrical conductor. | Salty water is an electrical conductor. |
Key Stage 3
Meaning
An electrical conductor is a material with a very low resistance to the flow of electricity.
About Electrical Conductors
- Metal elements are good electrical conductors.
- Metals make good conductors because they have free electrons that can move around the metal.
- Non-metal elements are usually poor electrical conductors. Carbon in the form of graphite is an exception to this.
- Salts that are molten or dissolved in water are electrical conductors.
- Salts make good conductors when the ions are free to move through the substance.
- To determine if an object is a good electrical conductor the object can be added to a circuit. If a current flows then it is a good conductor.
- To compare the conductivity of different objects an ammeter can be added to the circuit. The higher the current the better the object is at conducting.
Key Stage 4
Meaning
An electrical conductor is a material with a very low resistance to the flow of electricity.
About Electrical Conductors
- Metal elements are good electrical conductors.
- Metals make good conductors due to the metallic bonds in which a sea delocalised electrons is free to move past a lattice of positive metal ions.
- Non-metal elements are usually poor electrical conductors as electrons are not free to move from one atom to another. Carbon in the form of graphite is an exception to this.
- Salts that are molten or dissolved in water are electrical conductors.
- Salts make good conductors when the ions are free to move through the substance.
- To determine if an object is a good electrical conductor the object can be added to a circuit. If the ratio of potential difference to current is low then it is a good conductor.
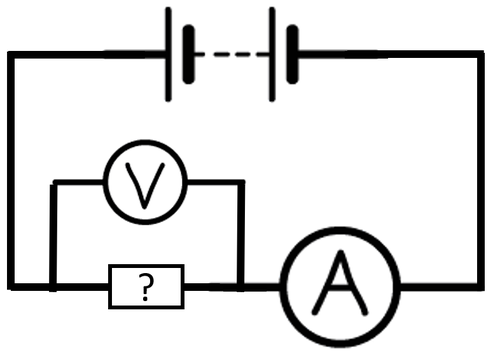
- To compare the conductivity of components in a circuit an ammeter can be added in series with the component and a voltmeter in parallel to the component. The smaller the resistance the better the object is at conducting.
| A circuit diagram showing how to find the resistance of an unknown component. |
References
AQA
- Conductor (electrical), page 48, GCSE Physics; Student Book, Collins, AQA
- Conductors; electrical, page 99, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA