Contents
Key Stage 3
Meaning
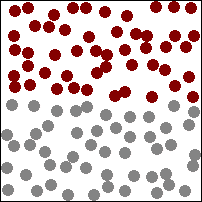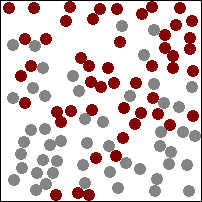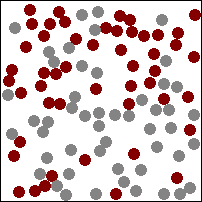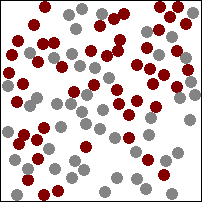
The higher the temperature the more the particles vibrate and faster they move in the material storing energy.
The Thermal Energy Store is the Energy Store associated with the temperature of an object.
About The Thermal Energy Store
- The hotter an object the more energy in the thermal energy store.
- The more mass an object has the more energy in its thermal energy store.
- Different materials can store different amounts of energy at the same temperature.
Examples
| The sparkler is at the highest temperature but it has the least thermal energy because it has so little mass. | These embers are much colder than a sparkler but have much more mass so they have more thermal energy. | The water in this hot tub is colder than both the sparkler and embers, but it has the most thermal energy because it has the most mass. |
Key Stage 4
Meaning
The Thermal Energy Store is the energy store associated with an objects temperature which is itself due to the random motion of particles within an object.
About the Thermal Energy Store
- Thermal Energy linked to the kinetic energy of the randomly moving particles inside an object.
- The thermal energy store of an object is related to three properties of the object:
- The mass of the object. - The greater the mass the greater the thermal energy of the object.
- The temperature of the object. - The greater the temperature the greater the thermal energy of the object.
- The specific heat capacity of the material which the object is made from. - The greater the Specific Heat Capacity the greater the thermal energy of the object.
Equation
Thermal Energy = Mass x (Specific Heat Capacity) x (Change in Temperature)
\(E_t = m c \Delta \theta\)
Where:
Et = Thermal Energy transferred to the object.
c = Specific Heat Capacity of the material which makes up the object
Δθ = Change in temperature of the object