Key Stage 2
Meaning
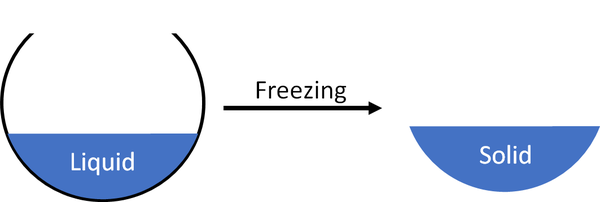
Freezing is when a liquid turns into a solid.
- Verb: To Freeze
- Present Participle: Freezing
When you cool a liquid:
|
|
| A liquid will freeze to become a solid.
|
About Freezing
- A liquid can freeze when its gets cold enough.
- Freezing is a reversible process. When a liquid freezes you can always melt it back into a liquid.
- You may have seen these liquids freeze:
|
|
|
| The water at this waterfall has frozen.
|
When the liquid wax drips down the side of a candle it freezes because it has cooled down.
|
Key Stage 3
Meaning
Freezing is an exothermic process in which a liquid turns into a solid.
About Freezing
- Freezing is a reversible process. When a liquid freezes you can always melt it back into a liquid.
- You can make a liquid freeze by cooling it.
Key Stage 4
Meaning
Freezing is an exothermic physical change in which a liquid turns into a solid.
About Freezing
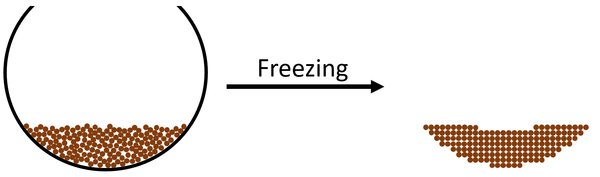
- Freezing happens when the particles in a liquid form bonds holding them in fixed positions as they lose potential energy.
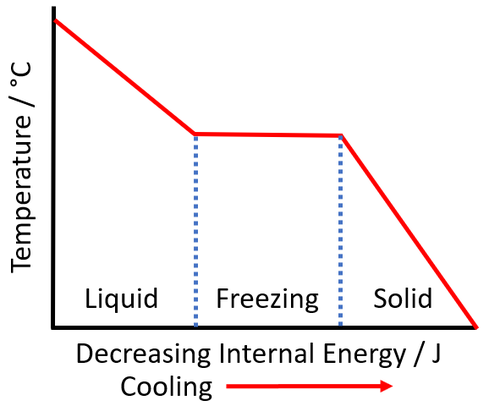
- The temperature at which a substance freezes is the same as the temperature at which it melts so it is the melting point.
- Freezing is an exothermic process, which means it emits energy when it takes place.
- Freezing is a physical change, which means it is reversible and does not produce new chemicals.
References
AQA
- Freezing, page 100, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Freezing, page 102, GCSE Chemistry, CGP, AQA
- Freezing, page 37, GCSE Chemistry; The Revision Guide, CGP, AQA
- Freezing, page 72, GCSE Physics, Hodder, AQA
- Freezing, pages 100-102, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Freezing, pages 110-112, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Freezing, pages 324, 328, GCSE Combined Science Trilogy 1, Hodder, AQA
- Freezing, pages 39, 40, GCSE Physics; The Revision Guide, CGP, AQA
- Freezing, pages 68-9, GCSE Chemistry; Student Book, Collins, AQA
- Freezing, pages 88-9, 102, GCSE Physics; Student Book, Collins, AQA
- Freezing; energy transfers, page 76, GCSE Physics, Hodder, AQA
Edexcel
- Freezing, page 35, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Freezing, page 98, GCSE Chemistry, CGP, Edexcel
- Freezing, page 98, GCSE Combined Science; The Revision Guide, CGP, Edexcel
OCR
- Freezing, page 12, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR
- Freezing, page 76, Gateway GCSE Chemistry, Oxford, OCR
- Freezing, pages 82, 152, 154, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR