Solid
Contents
Key Stage 2
Meaning
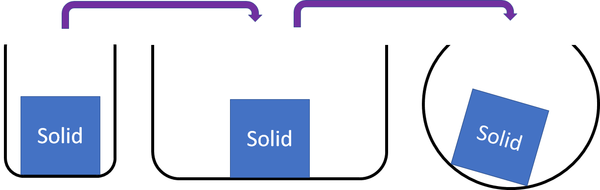
Solid is a state of matter that holds its shape and cannot be squashed into a smaller space.
About Solids
- Solids can be described with texture.
|
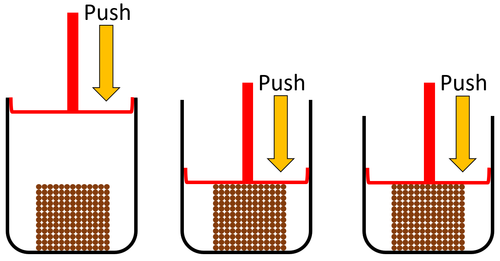
| Solids cannot be squashed into a smaller size. You can change their shape by squashing, but their size stays the same. |
Examples of solid materials:
- Brick
- Wood
- Plastic
- Glass
- Ice
Key Stage 3
Meaning
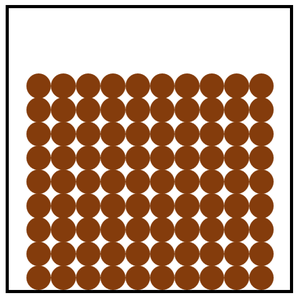
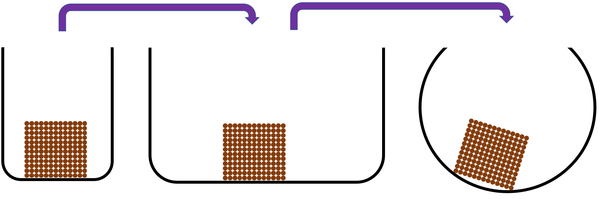
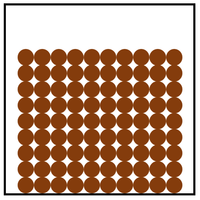
Solid is a state of matter where all the particles are in fixed positions, are touching and are in a regular arrangement.
About Solids
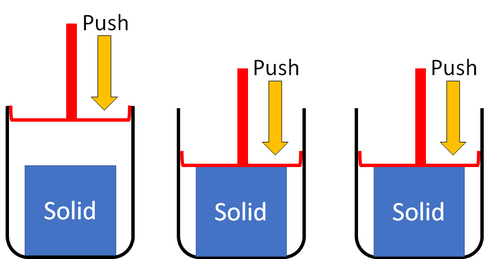
| Solids cannot be compressed into a smaller volume because the particles are already touching so they can't get any closer together. |
Key Stage 4
Meaning
Solid is a state of matter where all the particles vibrate around fixed positions.
About Solids
- When a substance is in its solid state it is usually more dense than in its liquid or gaseous state. Ice is an exception to this due to how the molecules arrange themselves, see Ice-Water Anomaly.
- A substance which is solid at room temperature has a larger force of attraction between particles than a substance which is liquid or gas at room temperature.
| Particle Diagram | Particle Arrangement | Property |
| Particles are in fixed positions. | Solids hold their shape. | |
| Convection cannot happen in solids. | ||
| Particles are very close together. | Solids cannot be compressed. | |
| Sound passes through solids faster than liquids and gases. | ||
| Particles vibrate. | ||
| Thermal Conduction happens best in solids. |
References
AQA
- Solid, pages 82-5, 100-1, GCSE Physics; Student Book, Collins, AQA
- Solids, page 107, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Solids, page 71, GCSE Physics, Hodder, AQA
- Solids, page 97, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Solids, pages 121, 122, 193, 195, GCSE Combined Science; The Revision Guide, CGP, AQA
- Solids, pages 164-5, 323, GCSE Combined Science Trilogy 1, Hodder, AQA
- Solids, pages 36, 37, 107, GCSE Chemistry; The Revision Guide, CGP, AQA
- Solids, pages 38-40, GCSE Physics; The Revision Guide, CGP, AQA
- Solids, pages 6, 36-37, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Solids, pages 76-79, 82, GCSE Physics; Third Edition, Oxford University Press, AQA
- Solids, pages 97, 100, 101, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Solids, pages 99, 102, 103, GCSE Chemistry, CGP, AQA
- Solids; density of, pages 321-2, 323, GCSE Combined Science Trilogy 1, Hodder, AQA
- Solids; density of, pages 69-70, GCSE Physics, Hodder, AQA
- Solids; density, page 108, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Solids; density, page 98, GCSE Combined Science Trilogy; Physics, CGP, AQA
Edexcel
- Solids, page 299, GCSE Physics, CGP, Edexcel
- Solids, pages 34, 35, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Solids, pages 95, 98, GCSE Chemistry, CGP, Edexcel
- Solids, pages 97, 98 GCSE Combined Science; The Revision Guide, CGP, Edexcel