Particle Model
Contents
Key Stage 3
Meaning
The particle model is a scientific theory that explains the properties of solids, liquids and gases by suggesting that all matter is made of particles, and that those particles behave differently in solids, liquids and gases.
About The Particle Model
- The particle model explains the properties of solids, liquids and gases.
- The particle model can explain changes of state.
- Evidence of the particle model can be shown by pouring 50ml of pure water and 50ml of pure ethanol into a measuring cylinder. The solution is only 97ml because ethanol molecules are bigger than water molecules so the water molecules fit between the ethanol molecules like pouring 50ml of sand and 50ml of marbles into the same container. It will not make 100ml.
- Evidence of the particle model can be shown by observing Brownian Motion.
Key Stage 4
Meaning
The particle model is a scientific theory that explains the properties of solids, liquids and gases by suggesting that all matter is made of particles, and that those particles behave differently in solids, liquids and gases.
About The Particle Model
- The particle model describes how the particles that make a solid, liquid or gas are arranged and how they move.
- In the particle model the particles are constantly moving and colliding with one another.
- The particles have kinetic energy which is passed on to each other during collisions.
| Diagram | Arrangement | Motion |
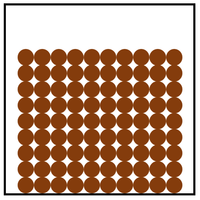
| In a solid the particles are in a regular arrangement and very close together. This is the most dense state of matter. | In a solid the particles vibrate around fixed positions. | |
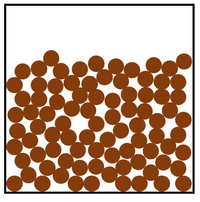
| In a liquid the particles are in a random arrangement with small gaps between them. | In a liquid the particles can slide past one another. | |
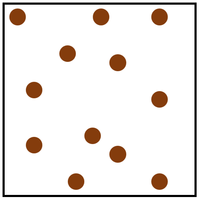
| In a gas the particles are in a random arrangement and spread far apart from one another. This is the least dense state of matter. | In a gas the particles are free to move in all directions. |
Limitations of the Particle Model
- The particle model is not a complete explanation for the properties of a material. However, it is a useful approximation which can make predictions about the properties of solids, liquids and gases, that is not perfect.
- The particle model only explains the properties of solids, liquids and gases but not why different materials are solid, liquid or gas at different temperatures.
The problems with the particle model are that it makes several assumptions which are not always the case:
| Assumption | Reality | Problem |
| Particles are spheres. | Particles are often molecules whose shape is not a sphere, some of which are long chains of atoms. |
|
| There are no forces between particles. | There are forces between atoms and intermolecular forces between molecules. |
|
| The size of all particles in a substance is the same. | A substance can be made of more than one different particle which have different sizes. |
Chemical Reactions in the Particle Model
- During a chemical reaction atoms are rearranged into new compounds. The particle model explains how temperature and pressure affect the rate of chemical reactions.
- In the particle model the particles collide with one another.
- If particles collide with enough kinetic energy it can break the chemical bonds holding the atoms to each other in a molecule.
- When collisions have caused molecules to break apart they can join back together in a new arrangement.
Temperature and Chemical Reactions
- When the temperature of a substance is increased it causes the particles to move around faster, with greater kinetic energy.
- As temperature increases the particles collide more often and with greater kinetic energy.
- When particles collide more often it means a greater chance of the chemical bonds breaking and atoms rearranging into new compounds.
- When particles have a large kinetic energy during a collision they are more likely to have enough energy to break the chemical bonds holding atoms in the molecules together.
Pressure and Chemical Reactions
- When the pressure of a substance is increased it causes the particles collide more often.
- When particles collide more often it means a greater chance of the chemical bonds breaking and atoms rearranging into new compounds.
References
AQA
- Particle model, pages 106, 107, 113-115, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Particle model, pages 36-37, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Particle model, pages 68-9, GCSE Chemistry; Student Book, Collins, AQA
- Particle model, pages 96, 97, 103, 104, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Particle; model, pages 82-5, 89-90, 96, 98, 175, GCSE Physics; Student Book, Collins, AQA
- Particle theory, pages 36, 37, GCSE Chemistry; The Revision Guide, CGP, AQA
- Particle theory, pages 97, 98, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Particle theory, pages 99, 100, GCSE Chemistry, CGP, AQA
Edexcel
- Particle model, pages 34, 35, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Particle model, pages 95-98, GCSE Chemistry, CGP, Edexcel
- Particle model, pages 97, 98, GCSE Combined Science; The Revision Guide, CGP, Edexcel
OCR
- Particle model, pages 18-21, Gateway GCSE Physics, Oxford, OCR
- Particle model, pages 18-23, 176-181, Gateway GCSE Chemistry, Oxford, OCR
- Particle model, pages 82, 107, 152, 155, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Particle theory, pages 12, 34, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR
- Particle theory, pages 14, 17, 18, Gateway GCSE Physics; The Revision Guide, CGP, OCR
Beyond the Curriculum
The Quantum Realm
While the particle model is a fantastic way to understand the behavior of matter at macroscopic scales, there's a whole new world waiting to be explored at the quantum level. At this minuscule scale, particles exhibit bizarre behaviors that challenge our everyday intuition.
Quantum Particles
In the quantum realm, particles are nothing like the neat spheres we often imagine in the particle model. They can exist in multiple places at once (a phenomenon known as superposition), and their properties can be interconnected through a phenomenon called entanglement. These behaviors are fundamental to the field of quantum physics and have practical applications in technologies like quantum computing.
Wave-Particle Duality
One of the most mind-boggling concepts is wave-particle duality. Particles, such as electrons and photons, can exhibit both wave-like and particle-like properties depending on how they are observed. This phenomenon challenges our understanding of the very nature of matter and is a cornerstone of quantum mechanics.
Subatomic Particles
In addition to the familiar protons, neutrons, and electrons, there's a whole zoo of subatomic particles out there. Some of these exotic particles, like quarks and neutrinos, play crucial roles in the fundamental forces that govern the universe.
Quarks - The Building Blocks
Quarks are the smallest known building blocks of matter. They combine in various ways to form protons, neutrons, and other particles. Understanding quarks and their interactions is a cutting-edge field in particle physics, delving deep into the structure of matter itself.
Neutrinos - Ghostly Particles
Neutrinos are fascinating because they interact very weakly with other matter. In fact, billions of neutrinos pass through your body every second without you even noticing. They are essential in astrophysics, helping us understand the inner workings of stars and supernovas.
Dark Matter and Dark Energy
As we explore the universe, we've discovered that ordinary matter, the kind made up of particles in the particle model, makes up only a small fraction of the cosmos. The majority of the universe is composed of mysterious substances known as dark matter and dark energy.
Dark Matter - The Invisible Force
Dark matter does not emit, absorb, or reflect light, making it completely invisible. Yet, its gravitational influence is unmistakable, holding galaxies together and shaping the cosmos. Understanding dark matter is a leading challenge in astrophysics.
Dark Energy - The Cosmic Accelerator
Dark energy is even more enigmatic. It's responsible for the accelerated expansion of the universe, a discovery that shook the world of physics. Its nature remains one of the greatest mysteries in modern science.
Particle Accelerators
To study particles at these tiny scales, scientists use colossal machines called particle accelerators. These devices can propel particles to near-light speeds, allowing us to recreate the extreme conditions of the early universe and discover new particles.
Large Hadron Collider (LHC)
The LHC, located beneath the Swiss-French border, is the most powerful particle accelerator on Earth. It played a pivotal role in the discovery of the Higgs boson, a particle that gives mass to other particles. Students with a passion for physics may dream of working on experiments like those conducted at the LHC.
The Unified Theory
Scientists are working tirelessly to create a unified theory that combines the laws of quantum mechanics and the theory of relativity. Such a theory would explain the behavior of particles on all scales, from the smallest quantum particles to the largest cosmic structures. It's a challenge that has intrigued physicists for generations.
Remember, these topics are beyond the scope of your curriculum, but they represent the frontiers of scientific research. If you're passionate about physics and enjoy exploring the mysteries of the universe, these are exciting areas to delve into further during your academic journey.