Key Stage 2
Meaning
A solution is a mixture where a substance is dissolved in a liquid.
About Solutions
- For most solutions the liquid is water.
- Some solids dissolve in water to make a solution.
- Once a substance is dissolved in water it is often not possible to see it anymore.
- If a substance has a colour before it is dissolved the solution be will that colour.
Examples
Key Stage 3
Meaning
- A solution is a mixture where a substance (usually a solid) is dissolved in a liquid.
About Solutions
- Solutions are usually made of a liquid that is known as a solvent and a solid which is known as a solute.
- When a solute dissolves in a solvent it becomes a solution.
- Some solutions are a mixture of two solvents, for example water and ethanol.
Examples
Separating Solutions
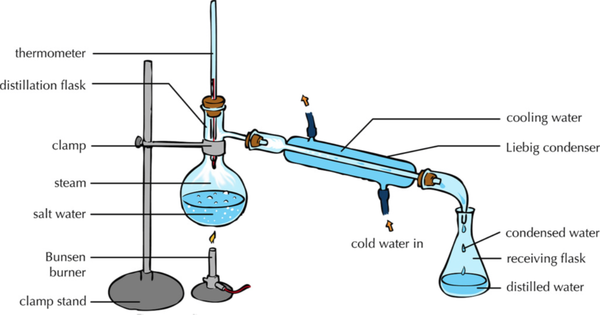
- The solute and solvent can be separated by distillation.
Key Stage 4
Meaning
- A solution is a mixture where a substance (usually a solid) is dissolved in a liquid.
About Solutions
- Solutions are usually made of a liquid that is known as a solvent and a solid which is known as a solute.
- When a solute dissolves in a solvent it becomes a solution.
- Some solutions are a mixture of two solvents, for example water and ethanol.
- Some gases may also dissolve in a liquid to make a solution. For example; Carbon Dioxide may dissolve in water. This makes the water acidic and is what leads to Ocean acidification if there is too much Carbon Dioxide in the atmosphere. A 'carbonated' beverage (like cola or lemonade) has Carbon Dioxide coming out of solution and creating bubbles. This is what makes the drink 'fizzy'.
References
AQA
- Solution, pages 112-3, GCSE Chemistry; Student Book, Collins, AQA
- Solutions, concentrations of, page 193, GCSE Combined Science Trilogy 1, Hodder, AQA
- Solutions, concentrations of, pages 85-7, GCSE Chemistry, Hodder, AQA
- Solutions; concentrations, pages 72-73, I GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Solutions; electrolysis, page 103, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Solutions; ionic compounds, pages 42-43, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Solutions; mass of solutes, page 73, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Solutions; titrations, pages 74-77, GCSE Chemistry; Third Edition, Oxford University Press, AQA
Edexcel
- Solutions, pages 6, 74, GCSE Chemistry, Pearson, Edexcel
- Solutions; concentrated, page 54, GCSE Chemistry, Pearson, Edexcel
- Solutions; dilute, page 54, GCSE Chemistry, Pearson, Edexcel
- Solutions; saturated, page 6, GCSE Chemistry, Pearson, Edexcel
OCR
- Solutions, pages 42-43, Gateway GCSE Chemistry, Oxford, OCR