PH Indicator
Contents
Key Stage 3
Meaning
A pH Indicator is a dye which changes colour depending on the pH of its environment.
About pH Indicators
- The colour of a pH indicator can be used to tell the pH of a solution.
- Different indicators will have a different range of colours for different pH values.
- A good pH indicator can be added to solution without affecting the pH of the solution. If a pH indicator changed the pH of a solution it could not give an accurate reading.
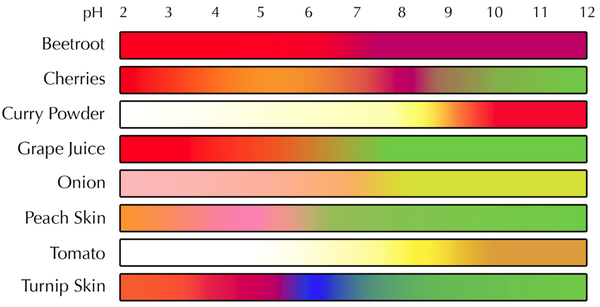
Some pH indicators you should know:
- Litmus Paper
- Red Cabbage Indicator
- Universal Indicator
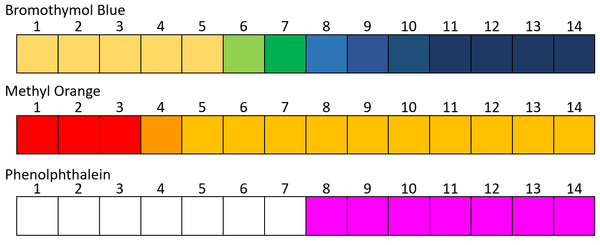
- Phenolphthalein
- Methyl Orange
- Bromothymol Blue
Examples
Key Stage 4
Meaning
A pH indicator is a dye which changes colour depending on the concentration of H+ or OH- ions in solution.
About pH Indicators
- pH indicators are used to determine the pH of a solution.
- Different pH indicators have a different range of effectiveness. Some only have two colours and change between them at a very specific pH. Others can have many different colours over a range of pH values.
References
AQA
- Indicators (of pH), page 289, GCSE Combined Science Trilogy; Biology, CGP, AQA
- Indicators (of pH), page 369, GCSE Biology, CGP, AQA
- Indicators, page 126, GCSE Biology; The Revision Guide, CGP, AQA
- Indicators, page 52, GCSE Physics; Third Edition, Oxford University Press, AQA
- Indicators, pages 124, 125, 237, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Indicators, pages 128, 134, GCSE Combined Science; The Revision Guide, CGP, AQA
- Indicators, pages 146, 147, 149, 150, 315, GCSE Chemistry, CGP, AQA