Specific Latent Heat of Vaporisation
Contents
Key Stage 4
Meaning
Specific Latent Heat of Vaporisation is the energy required to change the state of 1kg of a substance from liquid to gas.
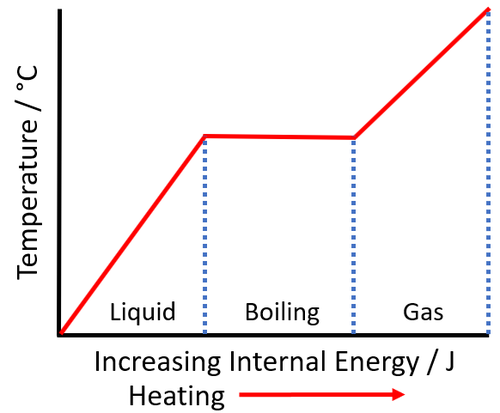
About Specific Latent Heat of Vaporisation
- The SI Unit of specific latent heat of vaporisation is the J/kg.
- Different materials have a different specific latent heat of vaporisation.
- Specific latent heat of vaporisation depends on the strength of the bonds holding the particles to each other in the liquid.
- The specific latent heat of vaporisation of a material can be found by measuring the energy needed to boil 1kg of the material.
| The increase internal energy during the time when the temperature remains constant is the energy required to vaporise the material. This can be used to calculate the specific latent heat of vaporisation. |
Equation
NB: You do not need to remember this equation but you need to be able to use it.
Specific Latent Heat = (Energy Transferred)/(Mass)
\(L_v = \frac{E}{m}\)
Where
\(L_v\) = The Specific Latent Heat of Vaporisation of the material.
\(E\) = The Energy transferred to the material while boiling.
\(m\) = The mass of the material.
Example Calculations
| 15g of Mercury requires 4.1kJ of energy to vaporise at its boiling point. Calculate the specific latent heat of vaporisation of the Mercury correct to two significant figures. | A pan containing 500g of water at 100°C is heated with an energy of 1.13kJ until all of the water has been vaporised. Calculate the specific latent heat of vaporisation of the water steam correct to two significant figures. |
| 1. State the known quantities in SI Units
E = 4.1kJ = 4.1x103J m = 15g = 15x10-3kg |
1. State the known quantities in SI Units
E = 1.13kJ = 1.13x103J m = 500g = 0.5kg |
| 2. Substitute the numbers into the equation and solve.
\(L_v = \frac{E}{m}\) \(L_v = \frac{4.1 \times 10^3}{15 \times 10^{-3}}\) \(L_v = 2.733 \times 10^5 J/kg\) \(L_v \approx 2.7 \times 10^5 J/kg\) |
2. Substitute the numbers into the equation and solve.
\(L_v = \frac{E}{m}\) \(L_v = \frac{1.13 \times 10^3}{0.5}\) \(L_v = 2260 J/kg\) \(L_v \approx 2300 J/kg\) |