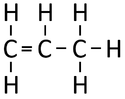Difference between revisions of "Propene"
(→About Propene) |
|||
| Line 10: | Line 10: | ||
==Key Stage 4== | ==Key Stage 4== | ||
===Meaning=== | ===Meaning=== | ||
| − | [[Propene]] is a [[gas]]eous ([[STP]]) [[ | + | [[Propene]] is a [[gas]]eous ([[STP]]) [[alkene]] with [[Chemical Formula|chemical formula]] C<sub>3</sub>H<sub>6</sub>. |
===About Propene=== | ===About Propene=== | ||
Revision as of 11:03, 3 April 2019
Key Stage 3
Meaning
Propene is a gaseous (at room temperature) hydrocarbon with chemical formula C3H6.
About Propene
- Propene is hydrocarbon because it contains only Hydrogen and Carbon atoms.
- Propene can be oxidised to produce Carbon Dioxide and Water.
- Propene + Oxygen → Carbon Dioxide + Water
Key Stage 4
Meaning
Propene is a gaseous (STP) alkene with chemical formula C3H6.
About Propene
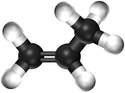
| Chemical Formula (CnH2n) | Structural Formula | Structural Diagram | Ball and Stick Model |
| C3H6 | CH2CHCH3 |
- Propene is hydrocarbon because it contains only Hydrogen and Carbon atoms.
- Propene has a double bond between two Carbon atoms.
- Propene is described as 'unsaturated' due to the double bond as it is not completely 'saturated' by Hydrogen atoms like Propane.
- Propene can be oxidised to produce Carbon Dioxide and Water.
- Propene + Oxygen → Carbon Dioxide + Water
- <chem>2C3H6 + 9O2 -> 6CO2 + 6H2O</chem>