Difference between revisions of "Indicator (Chemistry)"
(→About Indicators) |
|||
| Line 12: | Line 12: | ||
*[[Universal Indicator]] | *[[Universal Indicator]] | ||
*[[Phenolphthalein]] | *[[Phenolphthalein]] | ||
| − | *[[Methyl | + | *[[Methyl Orange]] |
*[[Bromothymol Blue]] | *[[Bromothymol Blue]] | ||
Revision as of 10:24, 29 September 2018
Key Stage 3
Meaning
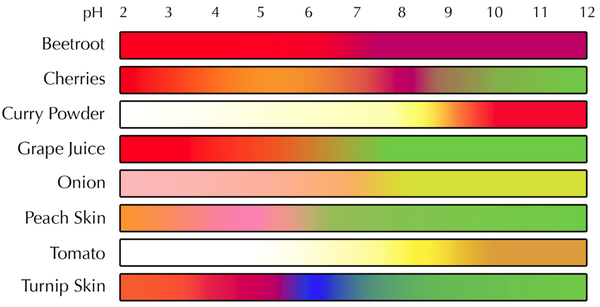
An indicator is a dye that changes colour depending on the pH of a solution.
About Indicators
- The colour of an indicator can be used to tell the pH of a solution.
- Different indicators will have a different range of colours for different pH values.
- A good indicator can be added to solution without affecting the pH of the solution. If an indicator change the pH of a solution it could not give an accurate reading.
Some indicators you should know:
- Litmus Paper
- Red Cabbage Indicator
- Universal Indicator
- Phenolphthalein
- Methyl Orange
- Bromothymol Blue