Difference between revisions of "Fullerenes"
(Created page with "==Key Stage 4== ===Meaning=== Fullerenes are allotropes of carbon in which the atoms are bonded in a hexagonal arrangement to form a sphere. {| c...") |
|||
| Line 1: | Line 1: | ||
==Key Stage 4== | ==Key Stage 4== | ||
===Meaning=== | ===Meaning=== | ||
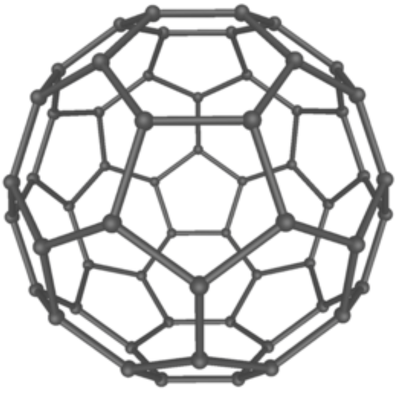
| − | [[Fullerenes]] are [[allotrope]]s of [[carbon]] in which the [[atom]]s are [[bond]]ed in a [[hexagon]]al | + | [[Fullerenes]] are [[allotrope]]s of [[carbon]] in which the [[atom]]s are [[bond]]ed in a mixture of [[hexagon]]al and [[pentagon]]al arrangements to form a [[sphere]]. |
{| class="wikitable" | {| class="wikitable" | ||
Revision as of 19:41, 31 December 2018
Key Stage 4
Meaning
Fullerenes are allotropes of carbon in which the atoms are bonded in a mixture of hexagonal and pentagonal arrangements to form a sphere.
| A diagram showing the arrangement of carbon atoms in a fullerene. |
About Fullerenes
- Fullerenes are a giant covalent structure.
- Fullerenes and any sphere shaped allotropes of carbon so they may have many different sizes and numbers of carbon atoms.