Difference between revisions of "Indicator (Chemistry)"
| (9 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
==Key Stage 3== | ==Key Stage 3== | ||
===Meaning=== | ===Meaning=== | ||
| − | An '''indicator''' is a [[dye]] that changes [[colour]] | + | An '''indicator''' is a [[dye]] that changes [[colour]] when other [[chemical]]s are present. |
===About Indicators=== | ===About Indicators=== | ||
| − | : The [[colour]] of | + | : Some '''indicators''' detect [[pH]] but others can be used to detect other [[chemical]]s. |
| + | Some non-pH '''indicators''' you should know: | ||
| + | *[[Iodine Solution]] - Used to detect starch. | ||
| + | *[[Biuret Solution]] - Used to detect proteins. | ||
| + | *[[Benedict's Test|Benedict's Solution]] - Used to detect simple [[sugar]]s like [[glucose]]. | ||
| + | *[[Limewater]] - Used to detect [[Carbon Dioxide]]. | ||
| + | |||
| + | ===About pH Indicators=== | ||
| + | : The [[colour]] of a '''pH indicator''' can be used to tell the [[pH]] of a [[solution]]. | ||
: Different '''indicators''' will have a different range of colours for different [[pH]] values. | : Different '''indicators''' will have a different range of colours for different [[pH]] values. | ||
: A good '''indicator''' can be added to [[solution]] without affecting the [[pH]] of the [[solution]]. If an '''indicator''' change the [[pH]] of a [[solution]] it could not give an [[accurate]] [[reading]]. | : A good '''indicator''' can be added to [[solution]] without affecting the [[pH]] of the [[solution]]. If an '''indicator''' change the [[pH]] of a [[solution]] it could not give an [[accurate]] [[reading]]. | ||
| + | Some '''pH indicators''' you should know: | ||
| + | *[[Litmus Paper]] | ||
| + | *[[Red Cabbage Indicator]] | ||
| + | *[[Universal Indicator]] | ||
| + | *[[Phenolphthalein]] | ||
| + | *[[Methyl Orange]] | ||
| + | *[[Bromothymol Blue]] | ||
===Examples=== | ===Examples=== | ||
| + | {| class="wikitable" | ||
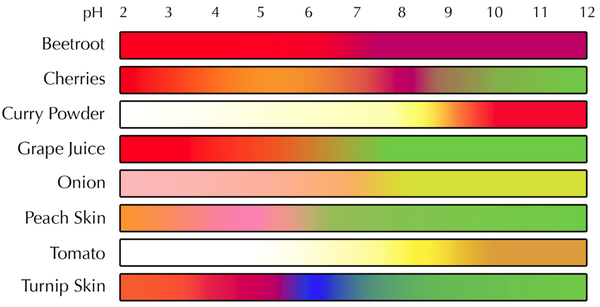
| + | |+ These are the colour ranges of different '''indicator''' plants. | ||
| + | |- | ||
| + | |[[File:NaturalIndicators.png|center|600px]] | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | An '''indicator''' is a [[dye]] that changes [[colour]] when other [[chemical]]s are present. | ||
| + | ===About Indicators=== | ||
| + | : Some '''indicators''' detect [[pH]] but others can be used to detect other [[chemical]]s. | ||
| + | Some non-pH '''indicators''' you should know: | ||
| + | *[[Iodine Solution]] - Used to detect starch. | ||
| + | *[[Biuret Solution]] - Used to detect proteins. | ||
| + | *[[Benedict's Test|Benedict's Solution]] - Used to detect simple [[sugar]]s like [[glucose]]. | ||
| + | *[[Limewater]] - Used to detect [[Carbon Dioxide]]. | ||
| + | |||
| + | ===About pH Indicators=== | ||
| + | : The [[colour]] of a '''pH indicator''' can be used to tell the [[pH]] of a [[solution]]. | ||
| + | : Different '''indicators''' will have a different range of colours for different [[pH]] values. | ||
| + | : A good '''indicator''' can be added to [[solution]] without affecting the [[pH]] of the [[solution]]. If an '''indicator''' change the [[pH]] of a [[solution]] it could not give an [[accurate]] [[reading]]. | ||
| + | Some '''pH indicators''' you should know: | ||
| + | *[[Litmus Paper]] | ||
| + | *[[Red Cabbage Indicator]] | ||
| + | *[[Universal Indicator]] | ||
| + | *[[Phenolphthalein]] | ||
| + | *[[Methyl Orange]] | ||
| + | *[[Bromothymol Blue]] | ||
| + | |||
| + | ===Examples=== | ||
{| class="wikitable" | {| class="wikitable" | ||
|+ These are the colour ranges of different '''indicator''' plants. | |+ These are the colour ranges of different '''indicator''' plants. | ||
| Line 16: | Line 62: | ||
|- | |- | ||
|} | |} | ||
| + | |||
| + | ===References=== | ||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Indicators (pH) page 52, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Indicators (pH), page 196, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Indicators, page 43, 65, 110, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Indicators, pages 105, 209, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''Indicators, pages 118-120, 131, 132, 185, 186, 205, 323, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945695/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945695&linkCode=as2&tag=nrjc-21&linkId=ceafcc80bcad6b6754ee97a0c7ceea53 ''Indicators, pages 112, 114, 220, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945679/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945679&linkCode=as2&tag=nrjc-21&linkId=a2db42f7b4bdf10cafaafa3bb9120940 ''Indicators, pages 43, 64, 81, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Indicators, pH, pages 112, 113, 119, 166, 199, 275, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
Latest revision as of 14:42, 13 December 2019
Contents
Key Stage 3
Meaning
An indicator is a dye that changes colour when other chemicals are present.
About Indicators
Some non-pH indicators you should know:
- Iodine Solution - Used to detect starch.
- Biuret Solution - Used to detect proteins.
- Benedict's Solution - Used to detect simple sugars like glucose.
- Limewater - Used to detect Carbon Dioxide.
About pH Indicators
- The colour of a pH indicator can be used to tell the pH of a solution.
- Different indicators will have a different range of colours for different pH values.
- A good indicator can be added to solution without affecting the pH of the solution. If an indicator change the pH of a solution it could not give an accurate reading.
Some pH indicators you should know:
- Litmus Paper
- Red Cabbage Indicator
- Universal Indicator
- Phenolphthalein
- Methyl Orange
- Bromothymol Blue
Examples
Key Stage 4
Meaning
An indicator is a dye that changes colour when other chemicals are present.
About Indicators
Some non-pH indicators you should know:
- Iodine Solution - Used to detect starch.
- Biuret Solution - Used to detect proteins.
- Benedict's Solution - Used to detect simple sugars like glucose.
- Limewater - Used to detect Carbon Dioxide.
About pH Indicators
- The colour of a pH indicator can be used to tell the pH of a solution.
- Different indicators will have a different range of colours for different pH values.
- A good indicator can be added to solution without affecting the pH of the solution. If an indicator change the pH of a solution it could not give an accurate reading.
Some pH indicators you should know:
- Litmus Paper
- Red Cabbage Indicator
- Universal Indicator
- Phenolphthalein
- Methyl Orange
- Bromothymol Blue
Examples
References
Edexcel
- Indicators (pH) page 52, GCSE Chemistry, Pearson, Edexcel
- Indicators (pH), page 196, GCSE Combined Science, Pearson Edexcel
- Indicators, page 43, 65, 110, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Indicators, pages 105, 209, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Indicators, pages 118-120, 131, 132, 185, 186, 205, 323, GCSE Chemistry, CGP, Edexcel