Difference between revisions of "Gas Pressure"
| Line 5: | Line 5: | ||
===About Gas Pressure=== | ===About Gas Pressure=== | ||
: '''Pressure in gases''' is caused by particles [[Collide|colliding]] with the [[object]] or walls of the container. | : '''Pressure in gases''' is caused by particles [[Collide|colliding]] with the [[object]] or walls of the container. | ||
| + | : Each time a [[particle]] in the [[gas]] hits an [[object]] or the walls it provides a [[force]]. | ||
| + | : The more [[particle]]s that hit the [[object]] or walls, the bigger the overall [[force]] it will experience. | ||
| + | : The faster the [[particle]]s hit the [[object]] or walls, the bigger the [[force]] that each [[particle]] [[collide]]s with. | ||
| + | |||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
Revision as of 13:08, 22 September 2018
Key Stage 3
Meaning
Gas pressure is the pressure on an object caused by a gas.
About Gas Pressure
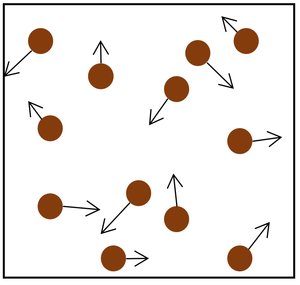
- Pressure in gases is caused by particles colliding with the object or walls of the container.
- Each time a particle in the gas hits an object or the walls it provides a force.
- The more particles that hit the object or walls, the bigger the overall force it will experience.
- The faster the particles hit the object or walls, the bigger the force that each particle collides with.
| The particle model of a gas showing the particles as red balls and their speed and direction shown by the arrows. The longer the arrows the faster they are moving. |
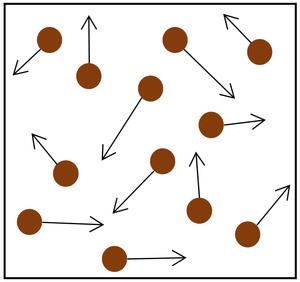
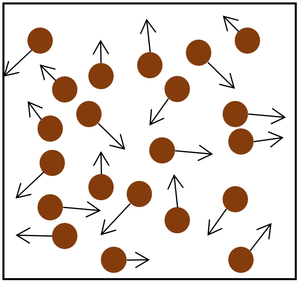
- The gas pressure can be increased in two ways:
| Increasing the temperature of the gas which makes the particles move faster. This causes the particles to hit the walls of the container more often and with a greater force. | Increasing the number of particles in the container. |