Difference between revisions of "Ethane"
(Created page with "Ethane is a gaseous (at room temperature) chemical compound with formula C<sub>2</sub>H<sub>6</sub>.") |
|||
| Line 1: | Line 1: | ||
| − | [[Ethane]] is a [[gas]]eous (at [[Room Temperature|room temperature]]) chemical [[ | + | ==Key Stage 3== |
| + | ===Meaning=== | ||
| + | [[Ethane]] is a [[gas]]eous (at [[Room Temperature|room temperature]]) [[hydrocarbon]] with [[Chemical Formula|chemical formula]] C<sub>2</sub>H<sub>6</sub>. | ||
| + | |||
| + | ===About Ethane=== | ||
| + | : [[Ethane]] is [[hydrocarbon]] because it contains only [[Hydrogen]] and [[Carbon]] [[atom]]s. | ||
| + | : [[Ethane]] can be [[oxidise]]d to [[product|produce]] [[Carbon Dioxide]] and [[Water]]. | ||
| + | : [[Ethane]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | ||
| + | : <chem>2C2H6 + 7O2 -> 4CO2 + 6H2O</chem> | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | [[Ethane]] is a [[gas]]eous (at [[Room Temperature|room temperature]]) [[hydrocarbon]] with [[Chemical Formula|chemical formula]] C<sub>2</sub>H<sub>6</sub>. | ||
| + | |||
| + | ===About Methane=== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | | style="height:20px; width:125px; text-align:center;" |[[Chemical Formula]] (C<sub>n</sub>H<sub>2n+2</sub>) | ||
| + | | style="height:20px; width:125px; text-align:center;" |[[Structural Formula]] | ||
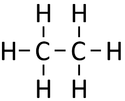
| + | | style="height:20px; width:125px; text-align:center;" |[[Structural Diagram]] | ||
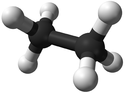
| + | | style="height:20px; width:125px; text-align:center;" |[[Ball and Stick Model]] | ||
| + | |- | ||
| + | | style="height:20px; width:125px; text-align:center;" |C<sub>2</sub>H<sub>6</sub> | ||
| + | | style="height:20px; width:125px; text-align:center;" |CH<sub>3</sub>CH<sub>3</sub> | ||
| + | |[[File:StructuralDiagramEthane.png|center|125px]] | ||
| + | |[[File:BallandStickEthane.png|center|125px]] | ||
| + | |} | ||
| + | |||
| + | : [[Ethane]] is [[hydrocarbon]] because it contains only [[Hydrogen]] and [[Carbon]] [[atom]]s. | ||
| + | : [[Ethane]] can be [[oxidise]]d to [[product|produce]] [[Carbon Dioxide]] and [[Water]]. | ||
| + | : [[Ethane]] + [[Oxygen]] → [[Carbon Dioxide]] + [[Water]] | ||
| + | : <chem>2C2H6 + 7O2 -> 4CO2 + 6H2O</chem> | ||
Revision as of 10:30, 3 April 2019
Key Stage 3
Meaning
Ethane is a gaseous (at room temperature) hydrocarbon with chemical formula C2H6.
About Ethane
- Ethane is hydrocarbon because it contains only Hydrogen and Carbon atoms.
- Ethane can be oxidised to produce Carbon Dioxide and Water.
- Ethane + Oxygen → Carbon Dioxide + Water
- <chem>2C2H6 + 7O2 -> 4CO2 + 6H2O</chem>
Key Stage 4
Meaning
Ethane is a gaseous (at room temperature) hydrocarbon with chemical formula C2H6.
About Methane
| Chemical Formula (CnH2n+2) | Structural Formula | Structural Diagram | Ball and Stick Model |
| C2H6 | CH3CH3 |
- Ethane is hydrocarbon because it contains only Hydrogen and Carbon atoms.
- Ethane can be oxidised to produce Carbon Dioxide and Water.
- Ethane + Oxygen → Carbon Dioxide + Water
- <chem>2C2H6 + 7O2 -> 4CO2 + 6H2O</chem>