Difference between revisions of "Density"
| Line 14: | Line 14: | ||
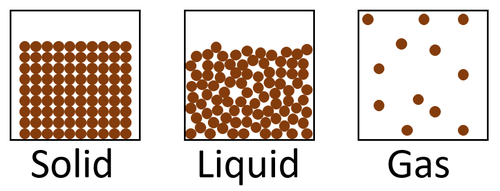
| style="height:20px; width:200px; text-align:center;" |[[Solid]]s are the most dense [[State of Matter|state of matter]] because there are a large number of [[particle]]s in a certain [[volume]] and [[gas]]es are the least '''dense''' [[State of Matter|state of matter]] because there are a small number of [[particle]]s in a the same [[volume]]. | | style="height:20px; width:200px; text-align:center;" |[[Solid]]s are the most dense [[State of Matter|state of matter]] because there are a large number of [[particle]]s in a certain [[volume]] and [[gas]]es are the least '''dense''' [[State of Matter|state of matter]] because there are a small number of [[particle]]s in a the same [[volume]]. | ||
|} | |} | ||
| + | |||
| + | ===Density and Floating=== | ||
| + | : If an [[object]] is more '''dense''' than [[water]] it will sink. | ||
| + | : If an [[object]] is less '''dense''' than [[water]] it will rise through [[water]] and float on the surface. | ||
===Equation=== | ===Equation=== | ||
| Line 22: | Line 26: | ||
: m = mass | : m = mass | ||
: V = volume | : V = volume | ||
| − | === | + | |
| − | : | + | ===Example Calculations=== |
| − | + | {| class="wikitable" | |
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''5000[[kg]] of [[Iron]] has a [[volume]] of 0.635m<sup>2</sup>. Calculate the density of [[Iron]].''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |Text | ||
| + | | style="height:20px; width:200px; text-align:center;" |Text | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" | | ||
| + | [[Mass]] = 5000[[kg]] | ||
| + | |||
| + | [[Volume]] = 0.635m<sup>3</sup> | ||
| + | |||
| + | :<math>\rho = \tfrac{m}{V}</math> | ||
| + | |||
| + | :<math>\rho = \tfrac{5000}{0.635}</math> | ||
| + | |||
| + | :<math>\rho = 7874kg/m<sup>3</sup></math> | ||
| + | | style="height:20px; width:200px; text-align:center;" |Text | ||
| + | | style="height:20px; width:200px; text-align:center;" |Text | ||
| + | |} | ||
Revision as of 11:05, 1 November 2018
Contents
Key Stage 3
Meaning
Density is the amount of mass per unit volume of an object.
About Density
- An object with a large amount of mass in a small volume is said to have a high density.
- An object with a small amount of mass spread over a large volume is said to have a low density.
- The units of density are kg/m3.
| Solids are the most dense state of matter because there are a large number of particles in a certain volume and gases are the least dense state of matter because there are a small number of particles in a the same volume. |
Density and Floating
- If an object is more dense than water it will sink.
- If an object is less dense than water it will rise through water and float on the surface.
Equation
- Density = Mass/volume
\[\rho = \tfrac{m}{V}\] Where:
- ρ = density
- m = mass
- V = volume
Example Calculations
| 5000kg of Iron has a volume of 0.635m2. Calculate the density of Iron. | Text | Text |
|
Volume = 0.635m3 \[\rho = \tfrac{m}{V}\] \[\rho = \tfrac{5000}{0.635}\] \[\rho = 7874kg/m<sup>3</sup>\] |
Text | Text |