Contents
Key Stage 3
Meaning
Density is the amount of mass per unit volume of an object.
About Density
- An object with a large amount of mass in a small volume is said to have a high density.
- An object with a small amount of mass spread over a large volume is said to have a low density.
- The units of density are kg/m3.
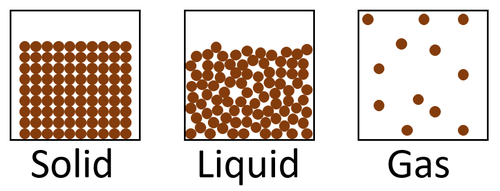
| Solids are the most dense state of matter because there are a large number of particles in a certain volume and gases are the least dense state of matter because there are a small number of particles in a the same volume. |
Density and Floating
- If an object is more dense than water it will sink.
- If an object is less dense than water it will rise through water and float on the surface.
Equation
- Density = Mass/volume
\[\rho = \tfrac{m}{V}\] Where:
Example Calculations
| 5000kg of Iron has a volume of 0.635m3. Calculate the density of Iron. | A 50,000cm3 container of water is full with a 50kg mass of water. Calculate the density of water. | A 200,000cm3 volume of air has a mass of 245g. Calculate the density of air. |
|
Volume = 0.635m3 \[\rho = \tfrac{m}{V}\] \[\rho = \tfrac{5000}{0.635}\] \[\rho = 7874kg/m<sup>3</sup>\] |
Volume = 50,000cm3 = 0.05m3 \[\rho = \tfrac{m}{V}\] \[\rho = \tfrac{50}{0.05}\] \[\rho = 1000kg/m<sup>3</sup>\] |
Volume = 200,000cm3 = 0.2m3 \[\rho = \tfrac{m}{V}\] \[\rho = \tfrac{0.245}{0.2}\] \[\rho = 1.225kg/m<sup>3</sup>\] |