Key Stage 4
Meaning
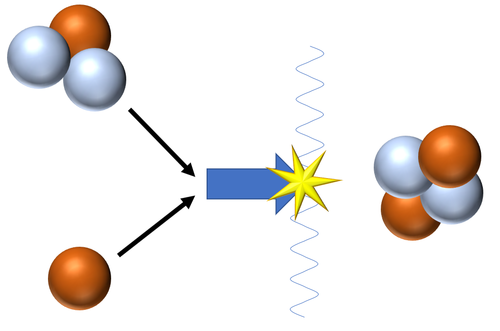
Nuclear fusion is a process in which two small atomic nuclei join together to become a larger nucleus.
About Nuclear Fusion
- In a nuclear fusion reaction the products have less mass than the reactants as some of the rest mass is converted into energy in the process.
- For a fusion reaction to occur the two nuclei must have enough kinetic energy to overcome the electrostatic force of repulsion between the positively charged nuclei.
- To provide enough kinetic energy to the nuclei the substance must be heated to a temperature of several million degrees Celsius.
- Nuclear fusion occurs naturally in the centre of a Star due to the high temperatures and pressure.
- Nuclear fusion is possible in laboratories on Earth but it cannot be sustained for long periods of time to produce enough energy to be useful as an energy resource.
- Nuclear fusion in laboratories on Earth must be done at much higher temperature than in the centre of Stars because the centre of Stars is a much higher pressure so nuclei collide more often.
Nuclear Fusion Reactions
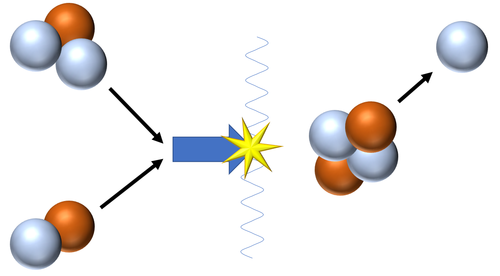
| Fusion of a Tritium and a Deuterium nucleus produces a Helium nucleus.
\({}_1^2H + {}_1^3H \rightarrow {}_2^4He + {}_0^1n\) This is the most common pathway for nuclear fusion in Stars as the neutron goes on to be captured by a Hydrogen nucleus to become Deuterium or captured by a Deuterium nucleus to become a Tritium nucleus. |
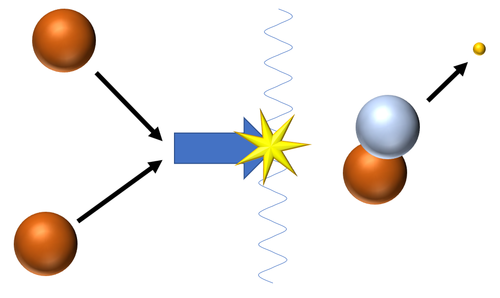
| Fusion of two Hydrogen nuclei (protons) produces a Deuterium nucleus.
\({}_1^1H + {}_1^1H \rightarrow {}_1^2H + {}_{-1}^0\beta\) |
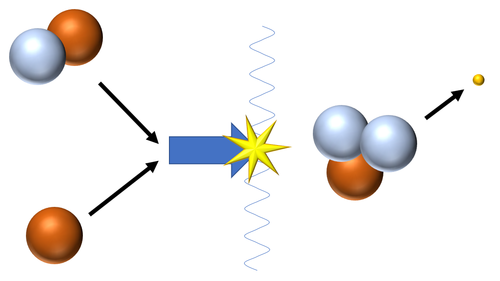
| Fusion of a Hydrogen nucleus (proton) and a Deuterium nucleus produces a Tritium nucleus.
\({}_1^1H + {}_1^2H \rightarrow {}_1^3H + {}_{-1}^0\beta\) |
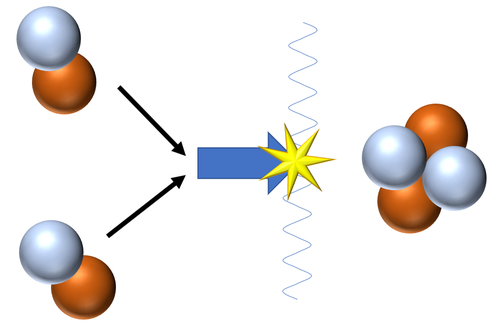
| Fusion of two Deuterium nuclei produces a Helium nucleus.
\({}_1^2H + {}_1^2H \rightarrow {}_2^4He\) |
| Fusion of a Hydrogen nucleus (proton) and a Tritium nucleus produces a Helium nucleus.
\({}_1^1H + {}_1^3H \rightarrow {}_2^4He\) |