Difference between revisions of "PH Indicator"
(Created page with "==Key Stage 3== ===Meaning=== A '''pH Indicator''' is a dye which changes colour depending on the pH of its environment. ===About pH Indicators=== : The colour of...") |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 6: | Line 6: | ||
: The [[colour]] of a '''pH indicator''' can be used to tell the [[pH]] of a [[solution]]. | : The [[colour]] of a '''pH indicator''' can be used to tell the [[pH]] of a [[solution]]. | ||
: Different '''indicators''' will have a different range of colours for different [[pH]] values. | : Different '''indicators''' will have a different range of colours for different [[pH]] values. | ||
| − | : A good '''indicator''' can be added to [[solution]] without affecting the [[pH]] of the [[solution]]. If | + | : A good '''pH indicator''' can be added to [[solution]] without affecting the [[pH]] of the [[solution]]. If a '''pH indicator''' changed the [[pH]] of a [[solution]] it could not give an [[accurate]] [[reading]]. |
Some '''pH indicators''' you should know: | Some '''pH indicators''' you should know: | ||
*[[Litmus Paper]] | *[[Litmus Paper]] | ||
| Line 18: | Line 18: | ||
{| class="wikitable" | {| class="wikitable" | ||
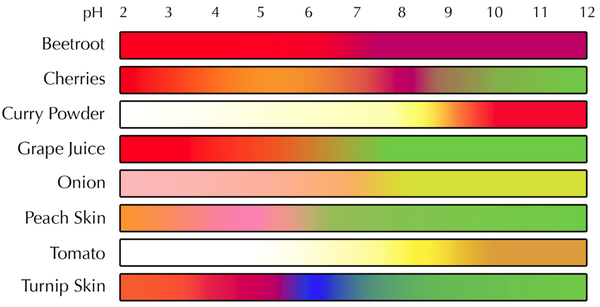
| − | |+ These are the colour ranges of different | + | |+ These are the colour ranges of different pH indicator plants. |
|- | |- | ||
|[[File:NaturalIndicators.png|center|600px]] | |[[File:NaturalIndicators.png|center|600px]] | ||
|- | |- | ||
|} | |} | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | A '''pH indicator''' is a [[dye]] which changes colour depending on the [[concentration]] of [[Hydrogen Ion (Chemistry)|H<sup>+</sup>]] or [[Hydroxide Ion (Chemistry)|OH<sup>-</sup> ions]] in [[solution]]. | ||
| + | |||
| + | ===About pH Indicators=== | ||
| + | : '''pH indicators''' are used to determine the [[pH]] of a [[solution]]. | ||
| + | : Different '''pH indicators''' have a different range of effectiveness. Some only have two colours and change between them at a very specific [[pH]]. Others can have many different colours over a range of [[pH]] values. | ||
| + | {| class="wikitable" | ||
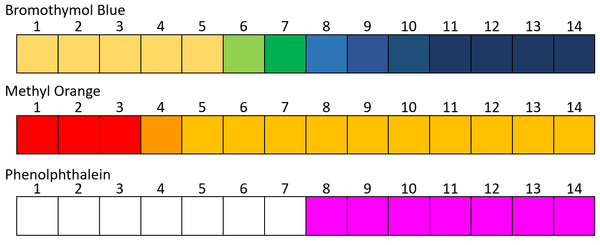
| + | |+ These are the colour ranges of some common pH indicators. | ||
| + | |- | ||
| + | |[[File:pHIndicators.png|center|600px]] | ||
| + | |} | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782946381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782946381&linkCode=as2&tag=nrjc-21&linkId=5ec5fc3f6429e30c1d9ab9bca2bccf93 ''Indicators (of pH), page 289, GCSE Combined Science Trilogy; Biology, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945954/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945954&linkCode=as2&tag=nrjc-21&linkId=100574c08fbbb64318256eb79ed61a76 ''Indicators (of pH), page 369, GCSE Biology, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945563/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945563&linkCode=as2&tag=nrjc-21&linkId=9a1d023a374038e6072f33c4f3cf808b ''Indicators, page 126, GCSE Biology; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/019835939X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=019835939X&linkCode=as2&tag=nrjc-21&linkId=57e96876985fc39b1a3d8a3e3dc238b6 ''Indicators, page 52, GCSE Physics; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Indicators, pages 124, 125, 237, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09996 ''Indicators, pages 128, 134, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Indicators, pages 146, 147, 149, 150, 315, GCSE Chemistry, CGP, AQA ''] | ||
| + | |||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782948120/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948120&linkCode=as2&tag=nrjc-21&linkId=dedef775c6a43dbb0a609441525adac0 ''pH indicators, page 317, GCSE Biology, CGP, Edexcel ''] | ||
Latest revision as of 02:22, 27 November 2019
Contents
Key Stage 3
Meaning
A pH Indicator is a dye which changes colour depending on the pH of its environment.
About pH Indicators
- The colour of a pH indicator can be used to tell the pH of a solution.
- Different indicators will have a different range of colours for different pH values.
- A good pH indicator can be added to solution without affecting the pH of the solution. If a pH indicator changed the pH of a solution it could not give an accurate reading.
Some pH indicators you should know:
- Litmus Paper
- Red Cabbage Indicator
- Universal Indicator
- Phenolphthalein
- Methyl Orange
- Bromothymol Blue
Examples
Key Stage 4
Meaning
A pH indicator is a dye which changes colour depending on the concentration of H+ or OH- ions in solution.
About pH Indicators
- pH indicators are used to determine the pH of a solution.
- Different pH indicators have a different range of effectiveness. Some only have two colours and change between them at a very specific pH. Others can have many different colours over a range of pH values.
References
AQA
- Indicators (of pH), page 289, GCSE Combined Science Trilogy; Biology, CGP, AQA
- Indicators (of pH), page 369, GCSE Biology, CGP, AQA
- Indicators, page 126, GCSE Biology; The Revision Guide, CGP, AQA
- Indicators, page 52, GCSE Physics; Third Edition, Oxford University Press, AQA
- Indicators, pages 124, 125, 237, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Indicators, pages 128, 134, GCSE Combined Science; The Revision Guide, CGP, AQA
- Indicators, pages 146, 147, 149, 150, 315, GCSE Chemistry, CGP, AQA