Flame Emission Spectroscopy
Key Stage 4
Meaning
Flame emission spectroscopy is a technique for identifying metals in a metal compound.
About Flame Emission Spectroscopy
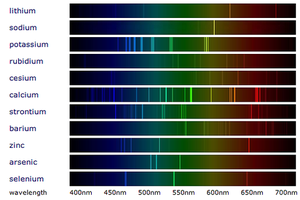
- Flame emission spectroscopy is an advanced version of the Flame Tests and uses a Spectroscope to separate the colours into a spectrum.
- When white light passes through a Spectroscope the colours are split into a spectrum (the rainbow). When metal compounds burn they only produce certain colours so when this light is passed through a spectroscope in flame emission spectroscopy it produces very specific lines called a 'Line Spectrum' instead of the broad spectrum seen from white light.
- Metals in a metal compound can be identified by comparing it to the line spectra of known metals.
References
AQA
- Flame emission spectroscopy, page 90, GCSE Chemistry; The Revision Guide, CGP, AQA
- Flame emission spectroscopy, pages 214-15, GCSE Chemistry, Hodder, AQA
- Flame emission spectroscopy, pages 262, 263, GCSE Chemistry, CGP, AQA
- Flame emission spectroscopy, pages 263, 284-5, GCSE Chemistry; Student Book, Collins, AQA