Metal
Contents
Key Stage 1
Meaning
Metal is a hard, smooth, shiny, bendy and opaque material.
About Metals
There are many different types of metal but they are all similar in their properties. Metals are used to make knives and forks because they are hard and smooth. Metals are used to make wires because they are bendy. Metal can be used to make hammers because it is hard and strong.
Key Stage 3
Meaning
A Metal is a material that is a good conductor of electricity and a good conductor of thermal energy.
About Metals
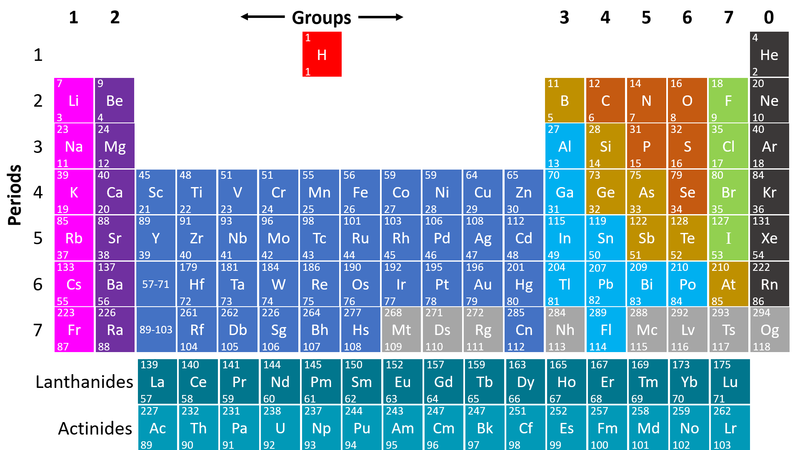
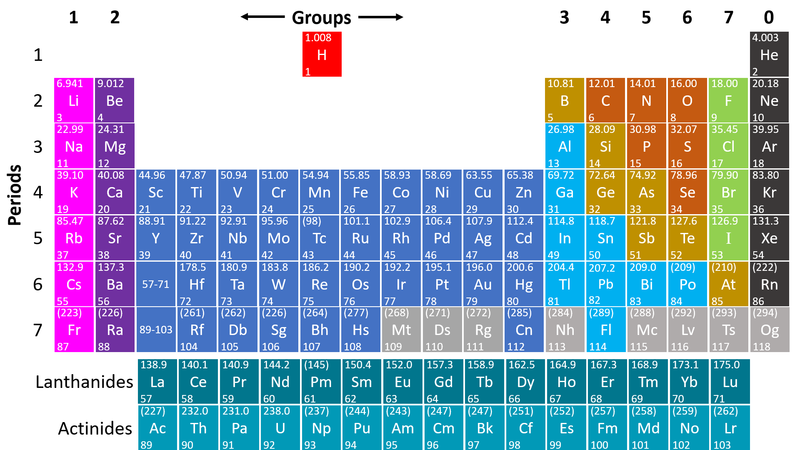
- Metals are found on the left hand side of the Periodic Table
| This Periodic Table shows the metal elements in blue and purple. |
Properties of Metals
There are several key properties of metals you should know. Metals are:
- Good conductors of electricity
- Good conductors of thermal energy
- Shiny - They have reflective surfaces when polished.
- Malleable - They can be hammered into shape.
- Ductile - They can be stretched into wires.
- Sonorous - They make a ringing sound when they are hit.
Key Stage 4
Meaning
A Metal is a a material that is a good conductor of electricity and a good conductor of thermal energy.
About Metals
- Metals are found on the left hand side of the Periodic Table
| This Periodic Table shows the metal elements in blue and purple. |
- Metals are on the left hand side of the Periodic Table because those elements lose electrons to form positive ions in compounds with non-metals.
- Metals form hydroxides when they react with water.
- Metal Oxides, Metal Carbonates and Metal Hydroxides all have pH above 7.
- Salts are metal compounds produced in Neutralisation reactions.
- Metal elements form metallic bonds in which positive ions are surrounded by a sea of delocalised electrons.
- A metal made of more than one metal element is called an alloy.
- Metals make good electrical and thermal conductors because the delocalised electrons are free to move around the material.
- Metals usually have high melting and boiling points making them solid at room temperature except for mercury which is a liquid at room temperature.
Applications and Properties
| Application | Properties |
| Cables | Strong - Does not break easily under a force. |
| Car bodies. | Malleable - can be hammered into shape. |
| Cables | Ductile - Can be stretched into wires. |
| Jet Engines | High Melting Point - Can be heated to high temperatures. |
| Electrical Wires | Good Electrical Conductor - electricity can pass through it easily. |
| Pots and pans | Good Thermal Conductor - Can be quickly heated. |
| Mirrors | Shiny - Reflects light. |
| Bells | Sonorous - Will make a sound when struck. |
References
AQA
- Metals, page 108, GCSE Combined Science; The Revision Guide, CGP, AQA
- Metals, page 45, GCSE Chemistry, Hodder, AQA
- Metals, pages 163-4, 247, GCSE Combined Science Trilogy 1, Hodder, AQA
- Metals, pages 23, 24, 35, 55, 56, 97, 98, GCSE Chemistry; The Revision Guide, CGP, AQA
- Metals, pages 24-27, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Metals, pages 56-7, 78-9, GCSE Chemistry; Student Book, Collins, AQA
Edexcel
- Metals, page 190, GCSE Combined Science, Pearson Edexcel
- Metals, pages 46, 203, GCSE Chemistry, Pearson, Edexcel
- Metals, pages 65, 66, 315, GCSE Chemistry, CGP, Edexcel
- Metals, pages 88, 123, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Metals; alloys, page 63, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Metals; alloys, pages 179, 180, GCSE Chemistry, CGP, Edexcel
- Metals; biological extraction methods, page 89, GCSE Chemistry, Pearson, Edexcel
- Metals; biological extraction, methods, page 233, GCSE Combined Science, Pearson Edexcel
- Metals; corrosion of, page 64, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Metals; corrosion of, pages 181, 182, GCSE Chemistry, CGP, Edexcel
- Metals; corrosion, page 235, GCSE Combined Science, Pearson Edexcel
- Metals; corrosion, page 91, GCSE Chemistry, Pearson, Edexcel
- Metals; electric currents, page 380, GCSE Combined Science, Pearson Edexcel
- Metals; electrical conductivity, page 191, GCSE Combined Science, Pearson Edexcel
- Metals; electrical conductivity, page 47, GCSE Chemistry, Pearson, Edexcel
- Metals; extraction as reduction, page 234, GCSE Combined Science, Pearson Edexcel
- Metals; extraction as reduction, page 90, GCSE Chemistry, Pearson, Edexcel
- Metals; extraction of, pages 155-160, GCSE Chemistry, CGP, Edexcel
- Metals; malleability, page 191, GCSE Combined Science, Pearson Edexcel
- Metals; malleability, page 47, GCSE Chemistry, Pearson, Edexcel
- Metals; ores, pages 232-233, GCSE Combined Science, Pearson Edexcel
- Metals; ores, pages 88-89, GCSE Chemistry, Pearson, Edexcel
- Metals; pages 25, 62, 73, 106, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Metals; properties, pages 190-191, GCSE Combined Science, Pearson Edexcel
- Metals; properties, pages 46-47, GCSE Chemistry, Pearson, Edexcel
- Metals; reaction with acids, page 210, GCSE Combined Science, Pearson Edexcel
- Metals; reaction with acids, page 66, GCSE Chemistry, Pearson, Edexcel
- Metals; reaction with carbonates, page 211, GCSE Combined Science, Pearson Edexcel
- Metals; reaction with carbonates, page 67, GCSE Chemistry, Pearson, Edexcel
- Metals; reactions of, pages 107, 114-116, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Metals; reactions of, pages 45, 52-54, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Metals; reactivity series, page 210, GCSE Combined Science, Pearson Edexcel
- Metals; reactivity series, page 66, GCSE Chemistry, Pearson, Edexcel
- Metals; reactivity, pages 148-151, GCSE Chemistry, CGP, Edexcel
- Metals; recycling, page 236, GCSE Combined Science, Pearson Edexcel
- Metals; recycling, page 92, GCSE Chemistry, Pearson, Edexcel
- Metals; structure and bonding, page 190, GCSE Combined Science, Pearson Edexcel
- Metals; structure and bonding, page 46, GCSE Chemistry, Pearson, Edexcel
- Metals; tests for, pages 95, 97, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Metals; transition metals, pages 177, 178, GCSE Chemistry, CGP, Edexcel
- Metals; uses, pages 104-105, GCSE Chemistry, Pearson, Edexcel
OCR
- Metals, pages 24, 25, 45, 55-57, 76-79, 87, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR
- Metals, pages 52-53, Gateway GCSE Chemistry, Oxford, OCR
- Metals, pages 96, 97, 113, 126, 127, 136, 137, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Metals; alloys, pages 40, 53, 214-215, Gateway GCSE Chemistry, Oxford, OCR
- Metals; corrosion of, page 79, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR
- Metals; corrosion, pages 216-219, Gateway GCSE Chemistry, Oxford, OCR
- Metals; displacement reactions, pages 142, 143, Gateway GCSE Chemistry, Oxford, OCR
- Metals; electrical conductivity, page 221, Gateway GCSE Chemistry, Oxford, OCR
- Metals; extraction of, pages 136, 137, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Metals; extraction of, pages 76, 77, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR
- Metals; formulae, page 86, Gateway GCSE Chemistry, Oxford, OCR
- Metals; Group 1 elements, pages 70, 71, 132-133, Gateway GCSE Chemistry, Oxford, OCR
- Metals; malleability, page 78, Gateway GCSE Chemistry, Oxford, OCR
- Metals; metallic bonding, pages 96, 97, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Metals; properties, pages 78, 215, 221, Gateway GCSE Chemistry, Oxford, OCR
- Metals; reaction with acids, pages 117, 142, 264-265, Gateway GCSE Chemistry, Oxford, OCR
- Metals; reactions with acids, page 113, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Metals; reactions with water, pages 132, 133, 141, 142, Gateway GCSE Chemistry, Oxford, OCR
- Metals; reactivity, pages 126, 127, 136, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Metals; reactivity, pages 70-71, 141-143, 206, 264-265, Gateway GCSE Chemistry, Oxford, OCR
- Metals; recycling, page 140, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Metals; recycling, page 85, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR
- Metals; recycling, pages 226,227, Gateway GCSE Chemistry, Oxford, OCR
- Metals; Resistance, pages 105, Gateway GCSE Physics, Oxford, OCR
- Metals; Specific heat capacity, pages 29, Gateway GCSE Physics, Oxford, OCR
- Metals; structures, pages 66-67, Gateway GCSE Chemistry, Oxford, OCR
- Metals; test for ions, page 60, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR
- Metals; transition, pages 88, 140-141, Gateway GCSE Chemistry, Oxford, OCR