Metallic Bond
Contents
Key Stage 4
Meaning
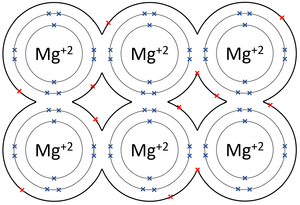
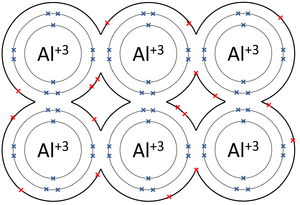
A metallic bond is type of chemical bond in which metal atoms are held together as a group of positive ions surrounded by a sea of delocalised electrons.
About Metallic Bonds
- Metallic bonds happen between metal atoms. This can be between atoms of the same element or different metal elements (an alloy).
- In metallic bonds the atoms form a lattice of positive ions surrounded by a sea of delocalised electrons.
- When metal atoms form metallic bonds they produce giant metallic structures but not simple compounds.
- More than one metal element in a metal is referred to as an alloy, not a compound.
Examples
| The outer shells of the Magnesium atoms overlap allowing the two electrons in each outer shell to move freely between atoms. | The outer shells of the Aluminium atoms overlap allowing the three electrons in each outer shell to move freely between atoms. |
References
AQA
- Metallic bond, pages 57-9, 66-7, GCSE Chemistry; Student Book, Collins, AQA
- Metallic bonding, pages 160-1, 163, GCSE Combined Science Trilogy 1, Hodder, AQA
- Metallic bonding, pages 52-55, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Metallic bonds, page 120, GCSE Combined Science; The Revision Guide, CGP, AQA
- Metallic bonds, pages 23, 35, GCSE Chemistry; The Revision Guide, CGP, AQA
Edexcel
- Metallic bonding, page 88, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Metallic bonding, pages 25, 63, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Metallic bonding, pages 65, 66, GCSE Chemistry, CGP, Edexcel