Fuel Cell
Contents
Key Stage 4
Meaning
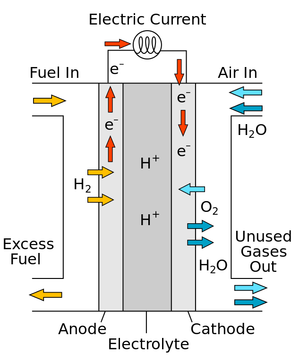
A diagram of a simple fuel cell.
A fuel cell is a device which can combine Hydrogen and Oxygen to produce a Potential Difference.
About Fuel Cells
- In a fuel cell Oxygen is combined with Hydrogen to produce Water.
- Fuel cells are designed to combine Hydrogen ions and Hydroxide ions to produce a potential difference between two electrodes.
- Fuel cells may be used in electric cars and were used on the Space Shuttle.
Advantages
- No Carbon Dioxide is produced.
- Refilling with Hydrogen is quicker than recharging a battery.
- They can be made in many different sizes for different uses.
Disadvantages
- Hydrogen must be stored as a Compressed Gas.
- Hydrogen is highly flammable.
- Hydrogen is made by electrolysis which requires electricity, which is often made by power stations burning fuel and producing Carbon Dioxide.
References
AQA
- Fuel cell, pages 172-3, 184-5, GCSE Chemistry; Student Book, Collins, AQA
- Fuel cells, page 65, GCSE Chemistry; The Revision Guide, CGP, AQA
- Fuel cells, pages 122-123, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Fuel cells, pages 138-9, GCSE Chemistry, Hodder, AQA
- Fuel cells, pages 190, 191, GCSE Chemistry, CGP, AQA
Edexcel
- Fuel cells, page 71, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Fuel cells, pages 124-125, GCSE Chemistry, Pearson, Edexcel
- Fuel cells, pages 206, 207, GCSE Chemistry, CGP, Edexcel