Electrolysis
Contents
Key Stage 3
Meaning
Electrolysis is a process where compounds are decomposed by an electrical current.
About Electrolysis
- Electrolysis can be used to extract metals from minerals when the metal is more reactive than Carbon.
Key Stage 4
Meaning
Electrolysis is a process in which an ionic compound is decomposed by passing an electrical current through it.
NB: Electrolysis is a GCSE Chemistry Required Practical: Electrolysis of a Solution
About Electrolysis
- In electrolysis two electrodes (an anode and a cathode) are placed in either a molten or aqueous ionic compound.
- Electrolysis cannot happen in solids because the ions vibrate around fixed positions in a solid and are not free to move.
- The positive ions are attracted to the negative electrode (cathode) and the negative ions are attracted to the positive electrode (anode).
- When the negative ions reach the anode they lose electrons to become atoms or neutral compounds.
- When the positive ions reach the cathode they gain electrons to become atoms. However, in aqueous solution if the metal ions are more reactive than Hydrogen then Hydrogen gas will be produced.
- To describe an electrolysis reaction half equations are used.
Examples
| Balanced Symbol Equation | 2Li2O(l) → 4Li(l) + O2(g) | CuCl2(aq) → Cu(s) + Cl2(g) | 2H2O(l) → 2H2(g) + O2(g) |
| Half Equation at cathode | Li+ + e- → Li | Cu+2(aq) + 2e- → Cu(s) | 2H+(aq) + 2e- → H2(g) |
| Half Equation at anode | 2O-2 → O2 + 4e- | 2Cl-(aq) → Cl2(g) + 2e- | 4OH- → 2H2O(l) + O2(g) + 4e- |
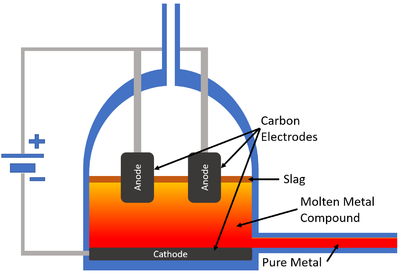
Electrolysis of Molten Ionic Compounds
- The electrolysis of molten ionic compounds decomposes those ionic compounds in a liquid state.
- The ionic compound must be heated and melted before electrolysis.
- Non-metal ionic elements or compounds will be collected at the anode where they lose their extra electrons.
- Metal ions will be collected at the cathode where they gain electrons.
| A diagram showing the electrolysis of a molten ionic compound in an electrolysis cell. |
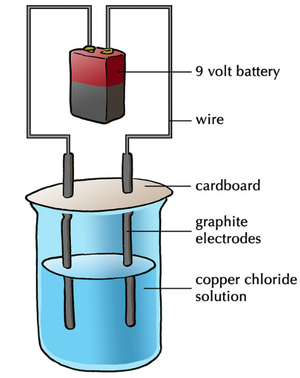
Electrolysis of Aqueous Ionic Compounds
- The electrolysis of aqueous ionic compounds decomposes those ionic compounds in solution.
- The electrolysis of aqueous ionic compounds will produce Hydrogen gas if the the metal elements is more reactive than Hydrogen.
- Non-metal ionic elements or compounds will be collected at the anode where they lose their extra electrons.
- Metal ions less reactive than Hydrogen will be collected at the cathode where they gain electrons.
- Metal ions more reactive than Hydrogen will displace Hydrogen atoms in the water after gaining [[electrons at the cathode. This causes Hydrogen gas to collect at the cathode.
References
AQA
- Electrolysis, extraction of copper, pages 200, GCSE Combined Science Trilogy 2, Hodder, AQA
- Electrolysis, page 117, GCSE Chemistry, Hodder, AQA
- Electrolysis, pages 102-111, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Electrolysis, pages 132, 133, 236, GCSE Combined Science; The Revision Guide, CGP, AQA
- Electrolysis, pages 141- 147, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Electrolysis, pages 166-172, GCSE Chemistry, CGP, AQA
- Electrolysis, pages 218-19, GCSE Combined Science Trilogy 1, Hodder, AQA
- Electrolysis, pages 58, 59, 109, GCSE Chemistry; The Revision Guide, CGP, AQA
- Electrolysis, pages 83, 130-1, 154-5, 333, GCSE Chemistry; Student Book, Collins, AQA
- Electrolysis; aqueous solutions, page 102, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Electrolysis; cell, pages 158-9, GCSE Chemistry; Student Book, Collins, AQA
- Electrolysis; half equations, page 256, GCSE Combined Science Trilogy 1, Hodder, AQA
- Electrolysis; half equations, pages 289-91, GCSE Chemistry, Hodder, AQA
- Electrolysis; metal extraction, pages 118-19, GCSE Chemistry, Hodder, AQA
- Electrolysis; metal extraction, pages 220-1, GCSE Combined Science Trilogy 1, Hodder, AQA
- Electrolysis; of aqueous ionic compounds, pages 119-21, GCSE Chemistry, Hodder, AQA
- Electrolysis; of aqueous ionic compounds, pages 221-3, GCSE Combined Science Trilogy 1, Hodder, AQA
- Electrolysis; of aqueous solutions with inert electrodes, pages 162-3, GCSE Chemistry; Student Book, Collins, AQA
- Electrolysis; of aqueous solutions, pages 145, 146, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Electrolysis; of aqueous solutions, pages 160-1, GCSE Chemistry; Student Book, Collins, AQA
- Electrolysis; of aqueous solutions, pages 170, 171, GCSE Chemistry, CGP, AQA
- Electrolysis; of lead bromide, page 142, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Electrolysis; of lead bromide, page 167, GCSE Chemistry, CGP, AQA
- Electrolysis; of molten ionic compounds, pages 117-18, GCSE Chemistry, Hodder, AQA
- Electrolysis; of molten ionic compounds, pages 156-7, GCSE Chemistry; Student Book, Collins, AQA
- Electrolysis; of molten ionic compounds, pages 219-20, GCSE Combined Science Trilogy 1, Hodder, AQA
- Electrolysis; of sodium chloride, page 146, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Electrolysis; of sodium chloride, page 171, GCSE Chemistry, CGP, AQA
Edexcel
- Electrolysis, page 224-225, GCSE Combined Science, Pearson Edexcel
- Electrolysis, pages 110-112, 118, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Electrolysis, pages 133-141, 157, 158, 182, GCSE Chemistry, CGP, Edexcel
- Electrolysis, pages 48-50, 56, 64, 111, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Electrolysis, pages 80-81, GCSE Chemistry, Pearson, Edexcel
- Electrolysis; aqueous solutions, pages 136-140, GCSE Chemistry, CGP, Edexcel
- Electrolysis; half equations, pages 134, 135, GCSE Chemistry, CGP, Edexcel
- Electrolysis; molten ionic substances, pages 134, 141, GCSE Chemistry, CGP, Edexcel
- Electrolysis; of copper sulfate, page 50, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Electrolysis; of metal ores, pages 157, 158, GCSE Chemistry, CGP, Edexcel
- Electrolysis; products, pages 228-229, GCSE Combined Science, Pearson Edexcel
- Electrolysis; products, pages 84-85, GCSE Chemistry, Pearson, Edexcel
- Electrolysis; salt solutions, pages 228-229, GCSE Combined Science, Pearson Edexcel
- Electrolysis; salt solutions, pages 84-85, GCSE Chemistry, Pearson, Edexcel