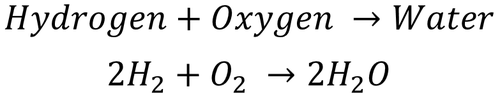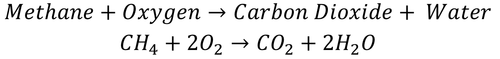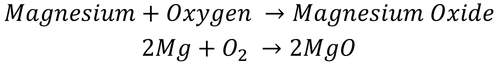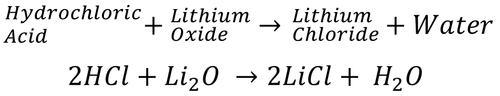Balanced Symbol Equation
Contents
Key Stage 3
Meaning
A Balanced Symbol Equation is a way to show the reactants and products in a chemical reaction using the chemical formulae of the reactants and products.
About Balanced Symbol Equations
- Balanced Symbol Equations shows the number of molecules of each chemical and the numbers of each atom within those chemicals.
- Balanced Symbol Equations can be used to find the quantity of each chemical needed to have a complete chemical reaction.
| This shows there are two H2 molecules and one O2 molecule needed to make two molecules of H2O. |
| This shows there is one CH4 molecule and two O2 molecules needed to make one molecule of CO2 and two molecules of H2O. |
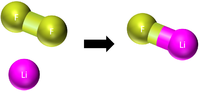
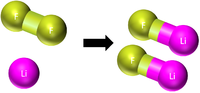
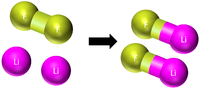
| This shows there are two atoms of Mg and one molecule of O2 needed to make two molecules of MgO. |
| This shows there are two HCl molecules and one Li2O molecule needed to make two molecules of LiCl and one molecule of H2O. |
Balancing Equations
- You may know the chemical formulae of the reactants and products but not know the number of each chemical needed. For that you must balance the equation.
- To balance and equation you need the same number of each type of atom on the left as you do on the right.
| Step 1 | Step 2 | Step 3 |
|
There are now too many Lithium atoms in the products so we need 2 in the reactants. |
This equation is balanced:
|
Aluminium + Chlorine → Aluminium Chloride
- _Al + _Cl2 → _AlCl3
- Reactants = Products
- 1Al = 1Al
- 2Cl ≠ 3Cl
We need the same number of Chlorine atoms on both sides. To do that we can find the Lowest Common Multiple. In this case it is 6, so we times the left 2 Chlorines by 3 and the right 3 Chlorines by 2.
- _Al + 3Cl2 → 2AlCl3
- Reactants = Products
- 1Al ≠ 2Al
- 6Cl = 6Cl
There are now too many Aluminium atoms on the right, so we need to multiply the left Aluminium by 2.
- 2Al + 3Cl2 → 2AlCl3
- Reactants = Products
- 2Al = 2Al
- 6Cl = 6Cl
This equation is now balanced: 2Al + 3Cl2 → 2AlCl3
Aluminium + Oxygen → Aluminium Oxide
- _Al + _O2 → _Al2O3
- Reactants = Products
- 1Al ≠ 2Al
- 2O ≠ 3O
We need the same number of Oxygen atoms on both sides. To do that we can find the Lowest Common Multiple. In this case it is 6, so we times the left 2 Oxygens by 3 and the right 3 Oxygens by 2.
- _Al + 3O2 → 2Al2O3
- Reactants = Products
- 1Al ≠ 4Al
- 6O = 6O
The Oxygen atoms are now balanced but the Aluminium atoms are not. We need 4 times as many Aluminium atoms on the left.
- 4Al + 3O2 → 2Al2O3
- Reactants = Products
- 4Al = 4Al
- 6O = 6O
This equation is now balanced: 4Al + 3O2 → 2Al2O3
Key Stage 4
Meaning
A Balanced Symbol Equation is a way to show the reactants and products in a chemical reaction using the chemical formulae of the reactants and products.
About Balanced Symbol Equations
- Balanced Symbol Equations show the number of molecules of each chemical and the numbers of each atom within those chemicals.
- Balanced Symbol Equations can be used to find the quantity of each chemical needed to have a complete chemical reaction.
Examples
2H2(g) + O2(g) → 2H2O(l)
C2H4(g) + Br2(g) → C2H4Br2(g)
C6H12O6(s) + 6O2(g) → 6H2O(l) + 6CO2(g)
4Al(s) + 3O2(g) → 2Al2O3(s)
Calculating the Mass Required for a Complete Reaction
|
Find the mass of Oxygen needed to completely oxidise all of the Magnesium: 2Mg + O2 → 2MgO 48g + x = y |
Find the mass of Oxygen needed for the complete combustion of Methane. CH4 + 2O2 → 2H2O + CO2 32g + x = y |
Find the mass of Hydrochloric Acid needed to completely neutralise all of the Sodium Hydroxide. NaOH + HCl → NaCl + H2O 20g + x = y + z |
|
Find the Relative Formula Mass of the reactants. Mr of Mg = 24g Mr of O2 = 16x2 Mr of O2 = 32g |
Find the Relative Formula Mass of the reactants. Mr of CH4 = 16g Mr of O2 = 16x2 Mr of O2 = 32g |
Find the Relative Formula Mass of the reactants. Mr of NaOH = 40g Mr of HCl = 36.5g |
|
Find the number of moles supplied of the known mass. No. Moles = \(\frac{Mass}{M_r}\) No. Moles = \(\frac{48}{24}\) No. Moles = 2 Mole Therefore 1 mole of O2 is needed. 1 mole of O2 = 32g |
Find the number of moles supplied of the known mass. No. Moles = \(\frac{Mass}{M_r}\) No. Moles = \(\frac{32}{16}\) No. Moles = 2 Mole Therefore 4 moles of O2 are needed. 4 moles of O2 = 128g |
Find the number of moles supplied of the known mass. No. Moles = \(\frac{Mass}{M_r}\) No. Moles = \(\frac{20}{40}\) No. Moles = 0.5 Mole Therefore 0.5 mole of HCl is needed. 0.5 mole of HCl = 18.25g |
Limiting Reactants
- A limiting reactant is a reactant that is not supplied in a large enough quantity for a complete reaction with the other reactants.
2H2(g) + O2(g) → 2H2O(l)
- If there is 4g of Hydrogen then 32g of Oxygen is needed for a complete reaction to occur.
- If there is 4g of Hydrogen but only 31g of Oxygen then the Oxygen is a limiting reactant.
- If there is 3g of Hydrogen but only 32g of Oxygen then the Hydrogen is a limiting reactant.
| If there are are 6g of Carbon and 15g of Oxygen in the following reaction:
C + O2 → CO2 Which is the limiting reactant in this reaction? |
If there are are 27g of Aluminium and 32g of Oxygen in the following reaction:
4Al + 3O2 → 2Al2O3 Which is the limiting reactant in this reaction? |
If there are are 180g of Glucose and 200g of Oxygen in the following reaction:
C6H12O6 + 6O2 → 6H2O + 6CO2 Which is the limiting reactant in this reaction? |
|
Find the Relative Formula Mass of the reactants. Mr of C = 12g Mr of O2 = 32g |
Find the Relative Formula Mass of the reactants. Mr of NaOH = 40g Mr of HCl = 36.5g |
Find the Relative Formula Mass of the reactants. Mr of NaOH = 40g Mr of HCl = 36.5g |
|
Find the number of moles supplied of each chemical. No. Moles of C = \(\frac{Mass}{M_r}\) No. Moles of C = \(\frac{6}{12}\) No. Moles = 0.5 mol Therefore 0.5 moles of O2 is needed. No. Moles of O = \(\frac{Mass}{M_r}\) No. Moles of O = \(\frac{15}{32}\) No. Moles = 0.46 mol Oxygen is the limiting reactant. 1 mole of O2 = 32g |
Find the number of moles supplied of each chemical. No. Moles of Al = \(\frac{Mass}{M_r}\) No. Moles of Al = \(\frac{27}{27}\) No. Moles = 1 mol Therefore 0.75 moles of O2 are needed. No. Moles of O = \(\frac{Mass}{M_r}\) No. Moles of O = \(\frac{32}{32}\) No. Moles = 1 mol Oxygen is the limiting reactant. |
Find the number of moles supplied of each chemical. No. Moles of C6H12O6 = \(\frac{Mass}{M_r}\) No. Moles of C6H12O6 = \(\frac{180}{180}\) No. Moles = 1 mol Therefore 6 moles of O2 are needed. No. Moles of O = \(\frac{Mass}{M_r}\) No. Moles of O = \(\frac{200}{32}\) No. Moles = 6.25 mol Glucose is the limiting reactant. |
Balancing Equations
- You may know the chemical formulae of the reactants and products but not know the number of each chemical needed. For that you must balance the equation.
- To balance and equation you need the same number of each type of atom on the left as you do on the right.
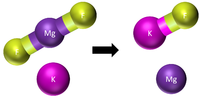
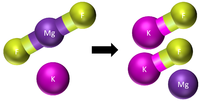
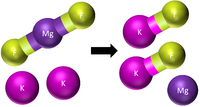
| Step 1 | Step 2 | Step 3 |
|
There are now too many Potassium atoms in the products so we need 2 in the reactants. |
This equation is balanced:
|
Balancing Equations with Ions
If the charge on an ion is known, this information can be used to find the chemical formula of an ionic compound and be used to balance a symbol equation.
- Group 1 Elements all form +1 ions; Li+1, Na+1, K+1
- Group 2 Elements all form +2 ions; Be+2, Mg+2, Ca+2
- Group 3 Elements all form +3 ions; Al+3
- Transition Metal Elements can form different ions which are shown by Roman Numerals;
Iron can form Fe (II) which is Fe+2 or Fe (III) is Fe+3, Manganese can form Mn (II) which is Mn+2 or Mn (IV) which is Mn+4
- Group 5 Elements all form -3 ions; N-3, P-3
- Group 6 Elements all form -2 ions; O-2, S-2
- Group 7 Elements all form -1 ions; F-1, Cl-1
- Some covalent compounds can form negative ions;
| Group 1 with Group 5 | Group 3 with Group 6 | Sulphates and Carbonates with Group 2 |
| Since Group 1 forms +1 ions and Group 5 forms -3 ions there will be 3 Group 1 ions for each Group 5 ion. | Since Group 3 forms +3 ions and Group 6 forms -2 ions there will be 2 Group 3 ions for every 3 Group 6 ions. | Sulphate ions and Carbonate ions both have a charge of -2 while Group 2 ions have a charge of +2 so there will be 1 Sulphate or Carbonate for every 1 Group 2 ion. |
|
Examples Li3N Na3P |
Examples B2S3 Al2O3 |
Examples MgSO4 CaCO 3 |
References
AQA
- Balanced equations, pages 6-7, 64-67, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Balancing equations, pages 15, 44, GCSE Chemistry; The Revision Guide, CGP, AQA
- Balancing equations, pages 181, 183-4, 250-1, GCSE Combined Science Trilogy 1, Hodder, AQA
- Balancing equations, pages 32, 33, 112, 113, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Balancing equations, pages 32, 33, 118, 119, GCSE Chemistry, CGP, AQA
- Balancing equations, pages 68, 70, 283-4, GCSE Chemistry, Hodder, AQA
- Balancing equations, pages 99, 199, GCSE Combined Science; The Revision Guide, CGP, AQA
- Balancing equations; deduction of balancing numbers, pages 188-9, GCSE Combined Science Trilogy 1, Hodder, AQA
- Balancing equations; ionic equations, pages 285-6, GCSE Chemistry, Hodder, AQA
- Balancing equations; molar ratios, pages 185-6, GCSE Combined Science Trilogy 1, Hodder, AQA
- Balancing numbers calculations, pages 73-4, GCSE Chemistry, Hodder, AQA
Edexcel
- Balanced equations, page 205, GCSE Combined Science, Pearson Edexcel'
- Balanced equations, page 61, GCSE Chemistry, Pearson, Edexcel'
- Balanced equations, pages 12, 31, 32, GCSE Chemistry; The Revision Guide, CGP, Edexcel'
- Balancing equations, pages 23-26, 89, 90, GCSE Chemistry, CGP, Edexcel
- Symbol equations, page 6, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Symbol equations, pages 31-33, GCSE Chemistry, CGP, AQA
- Symbol equations, pages 31-33, GCSE Combined Science Trilogy; Chemistry, CGP, AQA