Transition Metal
Contents
Key Stage 4
Meaning
Transition Metals (also known as transition elements) are a block of elements on the Periodic Table between Group 2 and Group 3.
About Transition Metals
- Transition Metals have the physical properties of metals.
- Transition Metals bond together with metallic bonds in which positive ions are surrounded by a sea of negatively charged electrons (known as delocalised electrons.
| Period 4 | 21Sc | 22Ti | 23V | 24Cr | 25Mn | 26Fe | 27Co | 28Ni | 29Cu | 30Zn |
| Period 5 | 39Y | 40Zr | 41Nb | 42Mo | 43Tc | 44Ru | 45Rh | 46Pd | 47Ag | 48Cd |
| Period 6 | 57La | 72Hf | 73Ta | 74W | 75Re | 76Os | 77Ir | 78Pt | 79Au | 80Hg |
| Period 7 | 89Ac | 104Rf | 105Db | 106Sg | 107Bh | 108Hs | 109Mt | 110Ds | 111Rg | 112Cn |
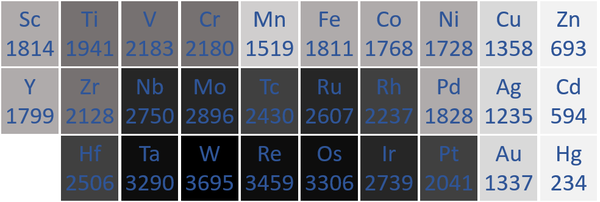
Melting Point
- Transition Metals usually have high melting points because metallic bonds are very strong, keeping the atoms vibrating in fixed positions.
| The transition metal melting points measured in Kelvin are written below each chemical symbol. |
| N.B. The Period 7 elements have not been included as they do not occur naturally and have not been made in large enough quantities to find their melting points. |
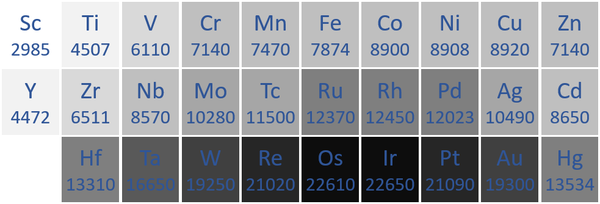
Density
- Transition Metals have a high density compared to other elements. However, many of the Actinides also have a density.
| The transition metal densities measured in kilograms per metre cubed are written below each chemical symbol. |
| N.B. The Period 7 elements have not been included as they do not occur naturally and have not been made in large enough quantities to find their [density|densities]]. |
Reactivity
- The Transition Metals are much less reactive than elements in group 1 and group 2.
- Most of the Transition Metals react slowly with Oxygen, Water and the Halogens at room temperature and some will not react at all.
- For combustion to occur the Transition Metals must be heated to very high temperatures. However, some Transition Metals will not combust at all.
Ion Formation
- Transition Metals form positive ions during chemical reactions.
- Transition Metals can form several different charge ions and these different charges can determine the physical properties of the compounds they are in. Specifically this affects the colour of those compounds.
In compounds the charge of the Transition Metal ion is given in Roman Numerals after the name of the metal
- Copper (I) Sulphate: +1 Ion of Copper giving the compound a light green colour.
- Copper (II) Sulphate: +2 Ion of Copper giving the compound a blue colour.
- Manganese (II) Chloride: +2 Ion of Manganese giving the compound a pale pink colour.
- Manganese (IV) Oxide: +4 Ion of Manganese giving it a very dark brown colour, often appearing black.
- Iron (II) Sulphate: *2 Ion of Iron giving the compound a pale green colour.
- Iron (III) Sulphate: *3 Ion of Iron giving the compound a redish-brown colour.
- Gem stones with various colours (red rubies, green emeralds, blue sapphires) get their colour from the Transition Metal ions they contain.
Catalysts
- Many Transition Metals can be used as catalysts for chemical reactions.
- Catalysts can increase the rate of reaction and lower the temperature needed to start a reaction.
- Catalysts are not used up in the chemical reaction so can be used over and over again.
- Transition Metal catalysts can increase the rate of reaction by either accepting or donating electrons so that not as much energy is needed for a reaction to happen.
These are some Transition Metal catalysts you may know:
- Iron: Used as a catalyst for Hydrogen and Nitrogen reacting to produce Ammonia.
- Platinum: Used in the production of Nitric Acid.
- Manganese: Used in the form of Manganese Dioxide as a catalyst for the decomposition of Hydrogen Peroxide.
References
AQA
- Transition metal, pages 48-9, GCSE Chemistry; Student Book, Collins, AQA
- Transition metals, pages 18-19, GCSE Chemistry, Hodder, AQA
- Transition metals, pages 23, 24, 25, GCSE Chemistry; The Revision Guide, CGP, AQA
Edexcel
- Transition metals, page 62, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Transition metals, pages 177, 178, GCSE Chemistry, CGP, Edexcel
- Transition metals, pages 96-97, GCSE Chemistry, Pearson, Edexcel
- Transition metals; chemical properties, page 97, GCSE Chemistry, Pearson, Edexcel
- Transition metals; physical properties, pages 96-97, GCSE Chemistry, Pearson, Edexcel