Group 1
Contents
Key Stage 4
Meaning
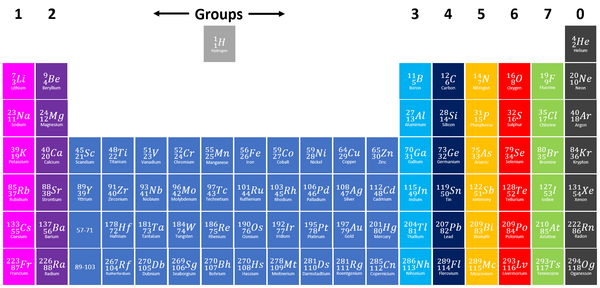
Group 1 elements, also known as Alkali Metals on the Periodic Table are the elements which have only one electron in their outer shell.
| Group 1 elements are shown in light purple at the far left of the Periodic Table. NB: Hydrogen is often shown in Group 1 due to having a single electron in the outer shell however it is not considered an Alkali Metal because it has very few properties in common with Alkali Metals. |
About the Alkali Metals
- Though Hydrogen has one electron in the outer shell it does not behave the same as other elements in group 1 so it is not considered to be an Alkali Metal.
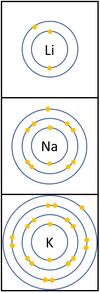
- The Alkali Metals have similar chemical properties because they all have one electron on their outer shell.
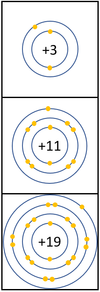
- Alkali Metals all produce ions with a +1 relative charge because they lose an electron in chemical reactions.
The Alkali Metals in order from least reactive to most reactive are:
Chemical Properties
- Alkali Metals are all highly reactive and will oxidise quickly in the presence of Oxygen.
- Alkali Metals all react strongly with water to produce metal hydroxides and Hydrogen gas.
- Alkali Metals all produce strong alkalis.
| In a chemical reaction the electron in the outer shell is lost.
The reactivity increases as you go down the group because:
|
Physical Properties
- Alkali Metals have a low density compared to other metals.
- Alkali Metals are all solid at room temperature but have a low melting point compared to other metals.
- Alkali Metals are soft and can be easily cut.
- Alkali Metals all appear shiny (before they oxidise).
References
AQA
- Alkali metals (Group 1), pages 14-15, GCSE Chemistry, Hodder, AQA
- Alkali metals, page 109, GCSE Combined Science; The Revision Guide, CGP, AQA
- Alkali metals, page 24, GCSE Chemistry; The Revision Guide, CGP, AQA
- Alkali metals, pages 129-31, GCSE Combined Science Trilogy 1, Hodder, AQA
- Alkali metals, pages 26-27, 30-31, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Alkali metals, pages 56-58, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Alkali metals, pages 56-59, 66, GCSE Chemistry, CGP, AQA
- Alkali metals, physical properties, page 162, GCSE Combined Science Trilogy 1, Hodder, AQA
- Group 1 (alkali metals), pages 129-31, GCSE Combined Science Trilogy 1, Hodder, AQA
- Group 1 (alkali metals), pages 42-3, 46, 49, 42-3, 131, 133-5, GCSE Chemistry; Student Book, Collins, AQA
- Group 1 (alkali metals); physical properties, page 162, GCSE Combined Science Trilogy 1, Hodder, AQA
- Group 1, page 109, GCSE Combined Science; The Revision Guide, CGP, AQA
- Group 1, page 24, GCSE Chemistry; The Revision Guide, CGP, AQA
- Group 1, pages 14-15, GCSE Chemistry, Hodder, AQA
- Group 1, pages 26-27, 30-31, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Group 1, pages 56-58, 66, GCSE Chemistry, CGP, AQA
- Group 1, pages 56-58, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
Edexcel
- Alkali metals, page 123, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Alkali metals, page 73, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Alkali metals, pages 128-129, GCSE Chemistry, Pearson, Edexcel
- Alkali metals, pages 242-243, GCSE Combined Science, Pearson Edexcel
- Alkali metals, pages 40, 49, 210-212, GCSE Chemistry, CGP, Edexcel
- Group 1, pages 20, 73, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Group 1, pages 40, 49, 210-212, GCSE Chemistry, CGP, Edexcel
- Group 1, pages 83, 123, GCSE Combined Science; The Revision Guide, CGP, Edexcel
OCR
- Alkali metals (Group 1 elements), pages 70, 71, 132-133, Gateway GCSE Chemistry, Oxford, OCR
- Alkali metals, page 121, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Alkali metals, page 51, GCSE Chemistry; The Revision Guide, CGP, OCR Gateway
- Group 1 elements, pages 70, 71, 132-133, Gateway GCSE Chemistry, Oxford, OCR
- Group 1, page 121, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Group 1, page 51, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR