Sodium
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
Sodium is a Group 1 element, on the Periodic Table, with an atomic number of 11.
About Sodium
Molecular Structure
- Sodium has the chemical symbol Na.
- Sodium atoms join together in large numbers to form a giant metal molecule.
Atomic Structure
- Sodium has 11 protons and 12 neutrons in its nucleus giving it an Atomic Number of 11 and an atomic mass of 23.
- An atom of Sodium has only 1 electron in its outer shell.
Properties
- Sodium is a more reactive alkali metal than Lithium but less reactive than Potassium.
- Sodium is more reactive than Carbon on the reactivity series so it must be extracted from its ore using electrolysis.
- Sodium oxidises quickly in the presence of Oxygen so it must be stored in oil.
- Sodium reacts strongly with water to produce Hydrogen gas and Sodium Hydroxide.
- Sodium is a solid at room temperature.
Key Stage 4
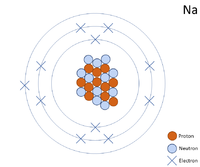
A 2 dimensional representation of the Bohr Model of a Sodium-23 isotope with 11 protons and 12 neutrons in the nucleus and 2 electrons in the first shell, 8 in the second and 1 in the outer shell.
Meaning
Sodium is a Group 1 element, on the Periodic Table, with 11 protons in the nucleus.
About Sodium
Molecular Structure
- Sodium has the chemical symbol Na.
- Sodium atoms join together in a giant metallic structure.
Atomic Structure
- The most stable isotope of Sodium has 12 neutrons in its nucleus giving it an atomic mass of 23.
- An atom of Sodium has only 1 electron in its outer shell.
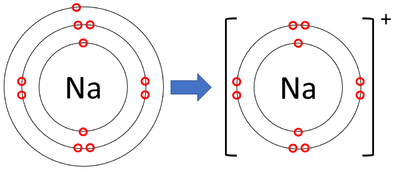
- Sodium ions have lost an electron to become positively charged.
| A diagram showing the formation of a Sodium ion. |
Properties
- Sodium is a more reactive alkali metal than Lithium but less reactive than Potassium.
- Sodium is more reactive than Carbon on the reactivity series so it must be extracted from its ore using electrolysis.
- Sodium oxidises quickly in the presence of Oxygen so it must be stored in oil.
- Sodium reacts strongly with water to produce Hydrogen gas and Sodium Hydroxide.
- Sodium is a solid at standard temperature and pressure with a melting point of 97.8 °C.