Key Stage 4
Meaning
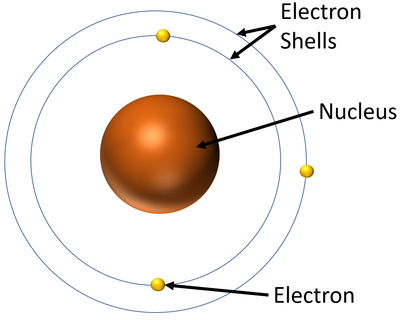
The Bohr Model is a model of the atom in which a positively charged nucleus is surrounded by electrons which orbit the nucleus in specific electron orbitals or 'shells'.
About the Borh Model
- The Bohr Model was developed by Neils Bohr following observations that electrons could gain or lose specific amounts of energy with the absorption and emission of light. This suggested that there were specific Energy Levels that electrons could be in. If an electron absorbs light it can move to a higher energy level and when it falls back to a lower energy level the electron will emit an electron.
- These energy levels are also referred to as Electron Orbitals and Electron Shells.
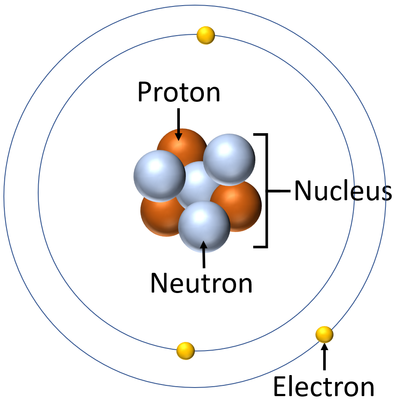
- The Bohr Model was updated after the discovery of the neutron in 1932 by James Chadwick. The new version of the model included the protons and neutrons within the nucleus.
References
AQA
- Bohr model of the atom, page 43, GCSE Chemistry, CGP, AQA
- Bohr model of the atom, page 104, GCSE Combined Science; The Revision Guide, CGP, AQA
- Bohr model of the atom, page 19, GCSE Chemistry; The Revision Guide, CGP, AQA
- Bohr model of the atom, page 342, GCSE Combined Science Trilogy 1, Hodder, AQA
- Bohr model of the atom, page 43, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Bohr’s model of the atom, page 95, GCSE Physics; Third Edition, Oxford University Press, AQA
- Bohr's model of the atom, page 43, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
Edexcel
- Bohr model, page 49, GCSE Physics; The Revision Guide, CGP, Edexcel
- Bohr model, pages 78, 172, GCSE Combined Science; The Revision Guide, CGP, Edexcel
OCR
- Bohr model of atom, pages 21, Gateway GCSE Physics, Oxford, OCR