Key Stage 3
Meaning
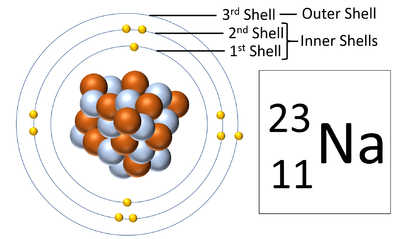
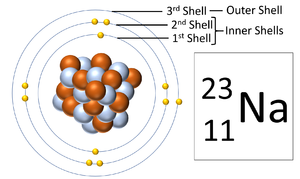
Electron shells are the places around a nucleus where an electron can orbit the nucleus.
About Electron Shells
- The number of electron shells is shown by the period on the Periodic Table.
- The number of electrons in the outer shell determines the chemical properties of the element.
Key Stage 4
Meaning
Electron orbitals, also known as an electron shells, are the locations where electrons orbit the nucleus of atoms.
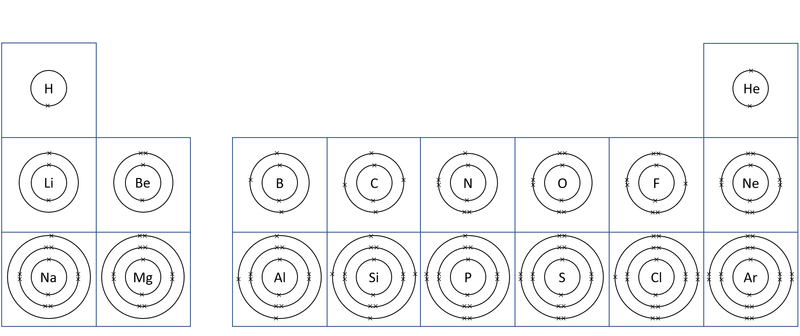
About Electron Orbitals
- Each electron orbital only holds a certain number of electrons.
- The first orbital can only hold a maximum of 2 electrons.
- The second orbital can hold a maximum of 8 electrons.
- The third orbital holds a maximum of 8 electrons.
- You do not need to know the numbers beyond this at Key Stage 4.
- These orbitals and the number of electrons in an atom determine the chemistry of an element.
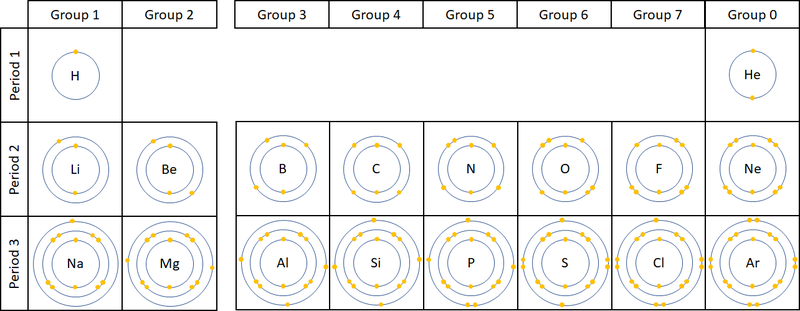
- The number of electron orbitals determines the Period on the Periodic Table.
- The number of electrons in the last orbital (Outer Shell) determines the Group on the Periodic Table.
- Atoms in the same group have similar chemical properties because they all have the same number of electrons in their Outer Shell.
References
AQA
- Shells, electrons, pages 13, 18-19, GCSE Chemistry; Third Edition, Oxford University Press, AQA
Edexcel
- Electron shells, page 94, GCSE Physics, Pearson Edexcel
- Electron shells, pages 16, 18, 19, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Electron shells, pages 162, 174, 358, GCSE Combined Science, Pearson Edexcel
- Electron shells, pages 18, 30, GCSE Chemistry, Pearson, Edexcel
- Electron shells, pages 33, 35, 42, 43, GCSE Chemistry, CGP, Edexcel
- Electron shells; outer, page 184, GCSE Combined Science, Pearson Edexcel
- Electron shells; outer, page 40, GCSE Chemistry, Pearson, Edexcel
OCR
- Electron shells, pages 13, 14, 16, 17, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR
- Electron shells, pages 21, Gateway GCSE Physics, Oxford, OCR
- Electron shells, pages 84, 87, 88, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR