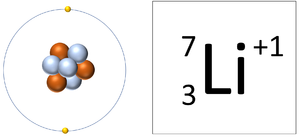Relative Atomic Charge
Key Stage 4
Meaning
Relative Atomic Charge is the charge of a particle compared to the charge of a single proton.
About Relative Atomic Charge
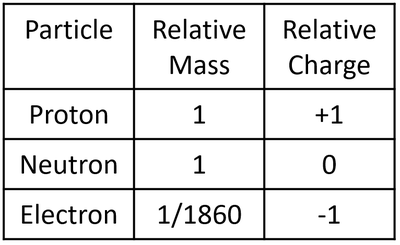
- Ions, nuclei and subatomic particles have extremely small charges such as the proton (1.6x10-19Coulombs) so instead of stating the charge in Coulombs it is compared to the charge of a proton.
| A table showing the relative mass and relative charge of the proton, neutron and electron. |
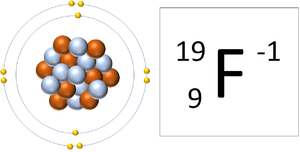
| A Lithium ion has 3 protons and 2 electrons so it has a positive charge. It now has a full Outer Shell. | A Fluorine ion has 9 protons and 10 electrons so it is negative charge. It now has a full Outer Shell. |