Fluorine
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
Fluorine is a Group 7 element, on the Periodic Table, with an atomic number of 9.
About Fluorine
Molecular Structure
- Fluorine has the chemical formula F2.
Atomic Structure
- Fluorine as 9 protons and 10 neutrons in its nucleus giving it an Atomic Number of 9 and an atomic mass of 19.
- An atom of Fluorine is missing one electron from having a full outer shell.
Properties
- Fluorine is a non-metal element.
- Fluorine is the most reactive Halogen.
- Fluorine reacts strongly with Hydrogen to produce Hydrogen Fluoride which dissolves in water to produce Hydrofluoric Acid.
- Fluorine is a strong bleaching agent.
- Fluorine kills bacteria.
- Fluorine is a yellow coloured gas at room temperature.
Key Stage 4
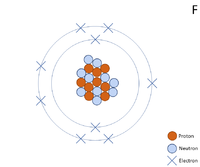
A 2 dimensional representation of the Bohr Model of a Fluorine-19 isotope with 9 protons and 10 neutrons in the nucleus and 2 electrons in the first shell and 7 in the outer shell.
Meaning
Fluorine is a Group 7 element, on the Periodic Table, with 9 protons in the nucleus.
About Fluorine
Molecular Structure
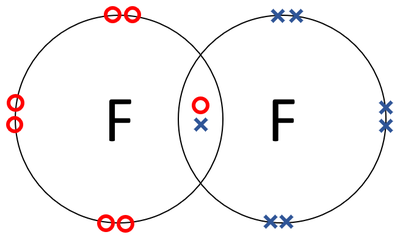
- Fluorine has the chemical formula F2.
- Fluorine atoms join together in a covalent bond.
| A dot and cross diagram of a Fluorine molecule. |
Atomic Structure
- The most stable isotope of Fluorine has 10 neutrons in its nucleus giving it an atomic mass of 19.
- An atom of Fluorine is missing one electron from having a full outer shell.
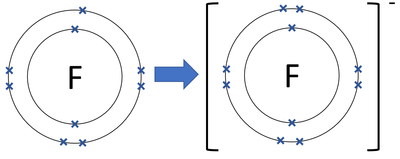
- Fluoride ions gain 1 electron to get a full outer shell and become negatively charged.
| A diagram showing the formation of a Fluoride ion. |
Properties
- Fluorine is a non-metal element.
- Fluorine is the most reactive Halogen.
- Fluorine reacts strongly with Hydrogen to produce Hydrogen Fluoride which dissolves in water to produce Hydrofluoric Acid.
- Fluorine is a strong bleaching agent.
- Fluorine kills bacteria.
- Fluorine is a yellow coloured gas at standard temperature and pressure.
Testing For Fluorine
- Collect the gas in a test tube.
- Place a piece of litmus paper over the mouth of the test tube.
- If the litmus paper is bleached white then the gas is Fluorine or Chlorine.