Catalyst
Contents
Key Stage 3
Meaning
A catalyst is a chemical which can make a chemical reaction happen more quickly, without being used up in the reaction.
About Catalysts
- A catalyst is neither are reactant nor a product in a reaction but it can make the reaction happen more quickly.
Examples
- Chlorophyll is a catalyst for the reaction that allows plants to turn Carbon Dioxide and Water into Glucose and Oxygen during photosynthesis.
- Manganese Dioxide is a catalyst that speeds up the break down of Hydrogen Peroxide into Water and Oxygen.
- Enzymes are a type of biological catalyst that can speed up reaction inside an organism.
Key Stage 4
Meaning
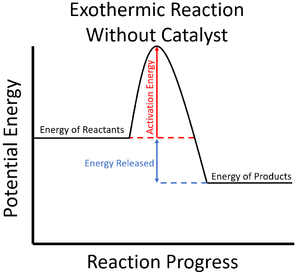
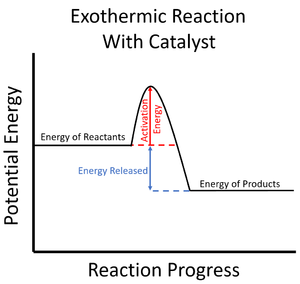
A catalyst is a chemical which can increase the rate of reaction and lower the activation energy for a particular reaction.
About Catalysts
- Catalysts provide a different "reaction pathway" for the reactants to form the products.
- Catalysts can lower the activation energy needed to start a Chemical Reaction.
| The activation energy is shown to be high in this reaction profile for an exothermic reaction without a catalyst. | The activation energy is shown to be low in this reaction profile for an exothermic reaction with a catalyst. |
- Since the particles in the reaction mixture will all have different amounts of energy (see Kinetic Theory), not all of them will have enough energy to react when they collide (see Collision Theory). Adding a catalyst lowers this activation energy so more particles have enough energy to react, increasing the rate of reaction.
- Enzymes are a type of biological catalyst that can increase the rate of reactions inside an organism.
References
AQA
- Catalyst; and dynamic equilibrium, page 164, GCSE Chemistry, Hodder, AQA
- Catalysts, page 168, GCSE Combined Science Trilogy 1, Hodder, AQA
- Catalysts, page 28, GCSE Biology; The Revision Guide, CGP, AQA
- Catalysts, pages 124-5, GCSE Combined Science Trilogy 2, Hodder, AQA
- Catalysts, pages 136-137, 159, 231, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Catalysts, pages 153-4, GCSE Chemistry, Hodder, AQA
- Catalysts, pages 166, 167, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Catalysts, pages 39, 68, 77, GCSE Chemistry; The Revision Guide, CGP, AQA
- Catalysts, pages 42-49, GCSE Biology; Third Edition, Oxford University Press, AQA
- Catalysts, pages 67, 198, 199, 302, GCSE Chemistry, CGP, AQA
- Catalysts; And dynamic equilibrium, pages 135, GCSE Combined Science Trilogy 2, Hodder, AQA
Edexcel
- Catalyst, pages 142-143, GCSE Chemistry, Pearson, Edexcel
- Catalyst; cracking page 160, GCSE Chemistry, Pearson, Edexcel
- Catalysts, page 13, GCSE Biology, Pearson, Edexcel
- Catalysts, page 133, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Catalysts, pages 13, 256-257, GCSE Combined Science, Pearson Edexcel
- Catalysts, pages 203, 238, 239, GCSE Chemistry, CGP, Edexcel
- Catalysts, pages 68, 82, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Catalysts; cracking, page 274, GCSE Combined Science, Pearson Edexcel