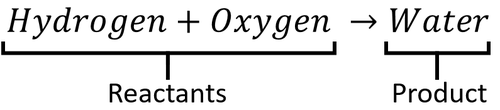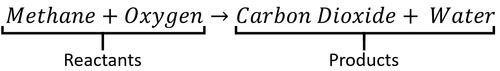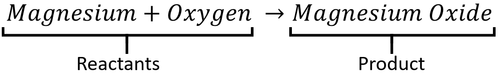Word Equation
Contents
Key Stage 3
Meaning
A word Equation is a way of representing chemical reactions to show the reactants and products using the names of the chemicals.
About Word Equations
- Word equations can be used to show the names of chemicals before and after reactions but they do not show the numbers of molecules or the number of atoms in each molecule. For that information you need a Balanced Symbol Equation.
Examples
Key Stage 4
Meaning
A word equation is a way of representing chemical reactions to show the reactants and products using the names of the chemicals.
About Word Equations
Examples
Combustion Reactions
These combustion reactions involve electron sharing.
- Methane + Oxygen → Carbon Dioxide + Water
- Ethane + Oxygen → Carbon Dioxide + Water
- Propane + Oxygen → Carbon Dioxide + Water
Oxidation Reactions
Oxidation reactions involve electron transfer.
- Magnesium + Oxygen → Magnesium Oxide
- Aluminium + Oxygen → Aluminium Oxide
- Iron + Oxygen → Iron Oxide
Displacement Reactions
Displacement reactions involve electron transfer.
- Copper Sulphate + Magnesium → Magnesium Sulphate + Copper
- Iron Chloride + Calcium → Calcium Chloride + Iron
- Lithium Hydroxide + Potassium → Potassium Hydroxide + Lithium
- Iron Oxide + Aluminium → Aluminium Oxide + Iron
Neutralisation Reactions
Neutralisation reactions involve proton transfer.
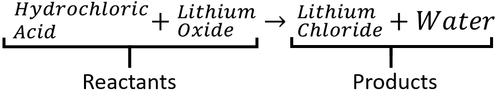
- Hydrochloric Acid + Lithium Oxide → Lithium Chloride + Water
- Hydrochloric Acid + Iron Oxide → Iron Chloride + Water
- Sulphuric Acid + Potassium Hydroxide → Potassium Sulphate + Water
- Sulphuric Acid + Aluminium Hydroxide → Aluminium Sulphate + Water
- Nitric Acid + Magnesium Carbonate → Magnesium Nitrate + Carbon Dioxide + Water
- Nitric Acid + Titanium Carbonate → Titanium Nitrate + Carbon Dioxide + Water
Thermal Decomposition Reactions
Thermal Decomposition reactions involve both electron sharing and electron transfer.
- Calcium Carbonate → Calcium Oxide + Carbon Dioxide
- Copper Carbonate → Copper Oxide + Carbon Dioxide
- Zinc Carbonate → Zinc Oxide + Carbon Dioxide
References
AQA
- Word equations, page 31, GCSE Chemistry, CGP, AQA
- Word equations, page 31, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Word equations, page 6, GCSE Chemistry; Third Edition, Oxford University Press, AQA
Edexcel
- Word equations, page 12, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Word equations, page 22, GCSE Chemistry, CGP, Edexcel
- Word equations, page 75, GCSE Combined Science; The Revision Guide, CGP, Edexcel