Calcium
Contents
Key Stage 2
Meaning
Calcium is a metal found in our bones.
Key Stage 3
Meaning
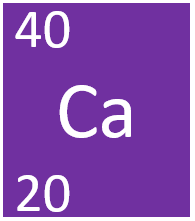
Calcium is a Group 2 element, on the Periodic Table, with an atomic number of 20.
About Calcium
Molecular Structure
- Calcium has the chemical Symbol Ca.
- Calcium atoms join together in large numbers to form a giant metal molecule.
Atomic Structure
- Calcium as 20 protons and 20 neutrons in its nucleus giving it an Atomic Number of 20 and an atomic mass of 40.
- An atom of Calcium has only 2 electrons in its outer shell.
Properties
- Calcium is a more reactive alkali earth metal than Magnesium but less reactive than Strontium.
- Calcium is more reactive than Carbon on the reactivity series so it must be extracted from its ore using electrolysis.
- Calcium reacts strongly with water to produce Hydrogen gas and Calcium Hydroxide and strongly with acid to produce Calcium salts.
- Calcium is a solid at room temperature.
Key Stage 4
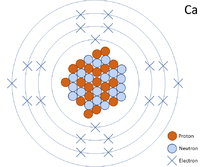
A 2 dimensional representation of the Bohr Model of a Calcium-40 isotope with 20 protons and 20 neutrons in the nucleus and 2 electrons in the first shell, 8 in the second, 8 in the third and 2 in the outer shell.
Meaning
Calcium is a Group 2 element, on the Periodic Table, with 20 protons in the nucleus.
About Calcium
Molecular Structure
- Calcium has the chemical symbol Ca.
- Calcium atoms join together in a giant metallic structure.
Atomic Structure
- The most stable isotope of Calcium has 20 neutrons in its nucleus giving it an atomic mass of 40.
- An atom of Calcium has only 2 electrons in its outer shell.
- Calcium ions have lost two electrons to become positively charged.
Properties
- Calcium is a more reactive alkali earth metal than Magnesium but less reactive than Strontium.
- Calcium is more reactive than Carbon on the reactivity series so it must be extracted from its ore using electrolysis.
- Calcium reacts strongly with water to produce Hydrogen gas and Calcium Hydroxide and strongly with acid to produce Calcium salts.
- Calcium is a solid at standard temperature and pressure.