Difference between revisions of "Half Life"
(→Finding Half Life and Count Rate) |
(→Finding Half Life and Count Rate) |
||
| Line 19: | Line 19: | ||
: The [[Count Rate|count rate]] of a sample of [[Unstable Isotope|unstable isotope]] can be predicted given its initial [[Count Rate|count rate]], its '''half life''' and the [[time]] it has been given. | : The [[Count Rate|count rate]] of a sample of [[Unstable Isotope|unstable isotope]] can be predicted given its initial [[Count Rate|count rate]], its '''half life''' and the [[time]] it has been given. | ||
| − | ===Finding Half Life and Count Rate=== | + | ===Finding Half Life and Count Rate from a Table=== |
{| class="wikitable" | {| class="wikitable" | ||
| style="height:20px; width:100px; text-align:center;" |Time /s | | style="height:20px; width:100px; text-align:center;" |Time /s | ||
Revision as of 12:21, 9 March 2019
Contents
Key Stage 4
Meaning
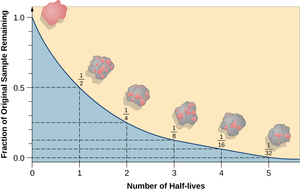
A half life is the time it takes for half of the unstable isotopes in a radioactive sample to decay.
About Half Life
- Every isotope has a unique half life.
- The half life of an isotope depends on how stable the isotope is.
- The half life of an isotope can be determined by measuring the emission of ionising radiation. The time it takes for the count rate of ionising radiation to halve, is the same as its half life.
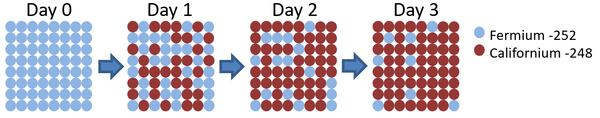
| This diagram shows the number of Fermium-252 atoms halves each day, so the half life is 1 day. |
- The half life of an isotope can be found from plotting a graph of the amount or isotope or the count rate against the time. The half life is the time these take to halve from their initial value.
- The count rate of a sample of unstable isotope can be predicted given its initial count rate, its half life and the time it has been given.
Finding Half Life and Count Rate from a Table
| Time /s | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| Count rate /Bq | 1000 | 707 | 500 | 353 | 250 | 176 | 125 | 88 | 62 |
1. Find the half life of this sample of radioactive isotope.
- The half life of of this sample of radioactive isotope can be found by halving the initial count rate.
- 1000/2 = 500Bq
- The isotope took 20 seconds to half in count rate:
- Half life = 20 seconds.
2. Predict the count rate at 90 seconds.
- Since the half life is 20 seconds the count rate 20 seconds earlier can be halved. :At 70 seconds the count rate was 88Bq. Therefore at 90 seconds the count rate would be:
- 88/2 = 44Bq
3. Predict what the count rate was 10 seconds before the sample was first tested.
- Since the half life is 20 seconds the count rate 20 seconds later can be doubled. :At 10 seconds the count rate was 707Bq. Therefore at -10 seconds the count rate would have been:
- 707x2= 1414Bq
| Time /min | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Count rate /Bq | 5000 | 3968 | 3150 | 2500 | 1984 | 1575 | 1250 | 992 | 788 |
1. Find the half life of this sample of radioactive isotope.
- The half life of of this sample of radioactive isotope can be found by halving the initial count rate.
- 5000/2 = 2500Bq
- The isotope took 3 minutes to half in count rate:
- Half life = 3 minutes.
2. Predict the count rate at 9 minutes.
- Since the half life is 3 minutes the count rate 3 minutes earlier can be halved.
- At 6 minutes the count rate was 1250Bq. Therefore at 9 minutes the count rate would be:
- 1250/2 = 625Bq
3. Predict what the count rate was 2 minutes before the sample was first tested.
- Since the half life is 3 minutes the count rate 3 minutes later can be doubled.
- At 1 minute the count rate was 3968Bq. Therefore at -2 minutes the count rate would have been:
- 3968x2= 7936Bq