Unstable Isotope
Key Stage 4
Meaning
Unstable Isotopes are isotopes which radioactively decay to form new stable isotopes of the same element or a different element.
About Unstable Isotopes
- An isotope may be unstable if:
- It has too many neutrons
- It has too few neutrons
- The nucleus is too massive.
- It has excess vibrational energy.
- An unstable isotope may decay causing it to transmute into a new element or a more stable isotope of the same element by releasing a particle from the nucleus.
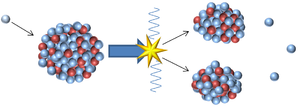
- An unstable isotope may be too massive so it can lose an alpha particle to become a less massive element or it can split into two smaller, more stable elements in a process called nuclear fission.
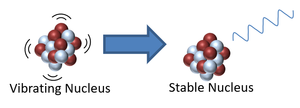
- An unstable isotope may be vibrating with excess energy so to lose this energy it will emit gamma radiation.
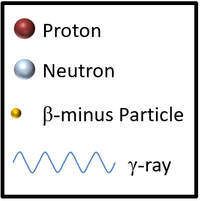
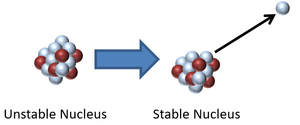
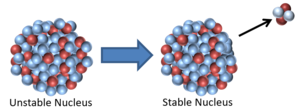
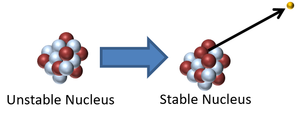
| This is a key to show the types of particles in the following decays of unstable nuclei. | This nucleus is unstable because it has too many neutrons relative to protons so it decays via neutron radiation reducing the atomic mass by 1. |
| This nucleus is unstable because it is too massive and has too few neutrons relative to protons so it decays via alpha emission reducing the atomic mass by 4 and the atomic number by 2. | This nucleus is unstable because it is too many neutrons so it decays via beta emission in which a neutron turns into a proton increasing the atomic number by 1. |
| This nucleus is unstable because it is has excess vibrational energy so it decays by emitting a gamma ray. After the decay it still has the same atomic mass and atomic number but is no longer vibrating. | This nucleus is unstable because it is far too massive and has too many neutrons so it splits into two smaller, more stable elements and releases some neutrons. This is not a radioactive decay and is referred to as nuclear fission. |