Neutron Radiation
Contents
Key Stage 4
Meaning
neutron radiation is a type of ionising radiation emitted from the nucleus of an unstable isotope.
About Neutron Radiation
- Neutron radiation is a neutron emitted from the nucleus of an unstable isotope.
- Neutrons have a relative atomic mass of 1 and relative charge of 0.
- Neutrons are emitted when the ratio of neutrons to protons is too large or when a massive unstable nucleus splits into two smaller nuclei in a process of nuclear fission.
Charge
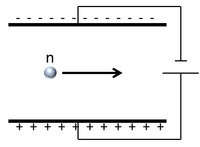
| Scientist were able to determine the charge of a neutron by sending it between two electrically charged plates and observing its path.
The neutron continues in a straight line so it must be neutral. |
Penetration Depth
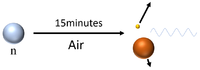
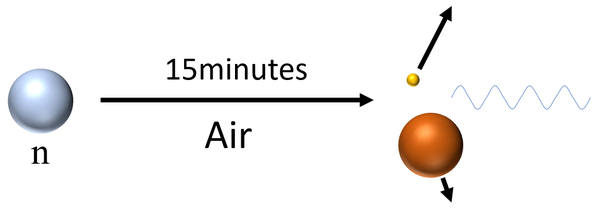
| Neutrons have a mean lifetime of around 15 minutes before they decay, so can travel several hundred kilometres depending on their velocity before they decay into a proton and a beta particle while emitting a gamma-ray. |
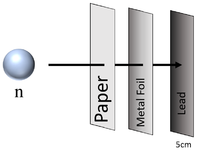
| Neutron radiation can penetrate paper and sheets of metal foil but cannot penetrate more than a few cm of Lead or a metre of concrete before they are captured by the nucleus of an atom. |
Ionising Potential
- With a charge of 0 and almost no effect on the electrons orbiting nuclei, neutrons are not directly ionising.
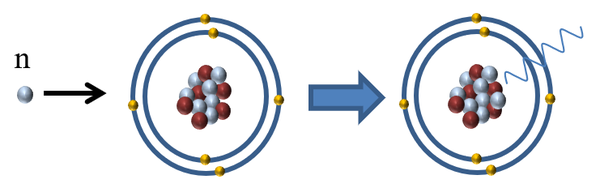
| Neutron radiation is referred to as indirectly ionising because it does not affect the electrons orbiting an atom but it can cause the release of directly ionising radiation in two ways: |
| It can be absorbed by a nucleus making it unstable and causing it to release a gamma-ray. |
| It can decay into a proton and a beta particle releasing a gamma-ray. |
Precautions
- Neutron radiation is not directly ionising but is as penetrating as gamma-rays.
- Neutron sources are kept inside a block of lead with a hole that only allows the neutrons out in one direction.
- Neutron radiation is too dangerous to handle directly so it must be done from behind a thick lead screen.
- Neutron emission is usually stimulated by bombarding nuclei with other particles. The neutron sources have a very short half life so they do not need to be kept in sealed Lead containers for long before they are no longer dangerously radioactive.
Applications
- Neutrons are used to stimulate nuclear fission in both nuclear reactors and nuclear bombs.
Equation
\({}_Z^AX \rightarrow {}_{Z}^{A-1}Y + {}_0^1n\)
\({}_{8}^{18}O \rightarrow {}_{8}^{17}O + {}_0^1n\)
Example Calculations
| Find the element 'X' and calculate its relative atomic mass 'A' and its relative atomic charge 'Z'.
\({}_{3}^{8}Li \rightarrow {}_{Z}^{A}X + {}_0^1n\) |
Find the element 'X' and calculate its relative atomic mass 'A' and its relative atomic charge 'Z'.
\({}_{Z}^{A}X \rightarrow {}_{10}^{26}Ne + {}_0^1n\) |
| 1. Calculate the relative atomic mass by looking at the top row of numbers.
8 = A + 1 A = 7 |
1. Calculate the relative atomic mass by looking at the top row of numbers.
A = 26 + 1 A = 27 |
| 2. Calculate the relative atomic charge by looking at the bottom row of numbers.
3 = Z + 0 Z = 3 |
2. Calculate the relative atomic charge by looking at the bottom row of numbers.
Z = 10 + 0 Z = 10 |
| 3. It's the same element but a different isotope.
\({}_{3}^{7}Li\) |
3. Since the relative atomic charge is the same as the atomic number look up the element on the periodic table.
\({}_{10}^{27}Ne\) |