Alpha Particle
Contents
Key Stage 4
Meaning
An alpha particle (α-particle) is a type of ionising radiation made of 2 protons and 2 neutrons emitted from the nucleus of an unstable isotope.
About Alpha Particles
- Alpha particles may also be referred to as alpha radiation and is written with the symbol α.
- Alpha particles are a Helium nucleus.
- Alpha particles have a relative atomic mass of 4 and relative charge of +2.
- Alpha particles are emitted when a nucleus is too large or the ratio of protons to neutrons is too large.
- Alpha particles are emitted rather than single protons or neutrons because the Helium nucleus is extremely stable.
Charge and Mass
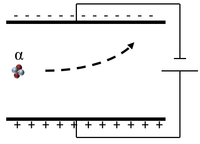
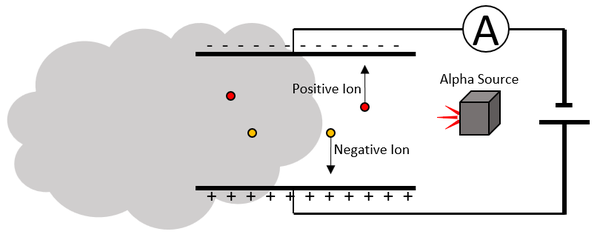
| Scientist were able to determine the charge and mass of an α-particle by sending it between two electrically charged plates and observing its path.
The α-particle moves towards the negative plate, so it must be positively charged. The rate of curvature depends on the mass:charge ratio which indicates it has a relative atomic mass of 4 and relative charge of +2. |
Penetration Depth
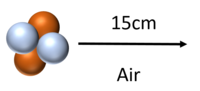
| Alpha particles can travel around 15cm through air (STP) before colliding with and ionising atoms or molecules. |
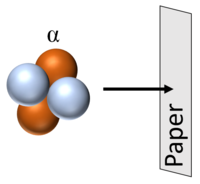
| Alpha particles can be stopped by a thin sheet of paper. |
Ionising Potential
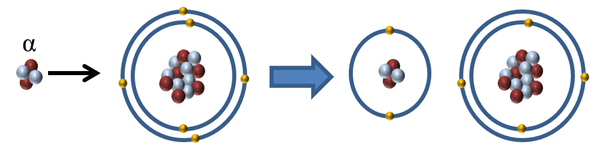
- With a charge of +2, α-particles are the most ionising of the three ionising radiations. It is capable of removing two electrons from a single atom or molecule or removing 1 electron from two atoms or molecules.
| When an α-particle interacts with an atom the α-particle can remove one or two electrons to ionise the atom. |
Precautions
- Alpha radiation is the most ionising but the least penetrating.
- Alpha sources are kept inside a block of lead with a hole that only allows the alpha particles out in one direction.
- Outside the body an organism can be protected from alpha radiation by keeping a distance greater than 5cm or by covering any bare skin.
- Inside an organism alpha particles are the most dangerous as they are highly ionising and there is nothing inside an organism to block them ionising biological molecules like DNA. Every precaution must be taken not to contaminate the skin with the alpha source in case it finds its way into the body through a cut or the mouth.
- When handling a source of alpha radiation the precautions which should be taken are:
- Wear gloves - to prevent contamination.
- Use tongs more than 5cm long to handle the source, never touch it - to prevent contamination and irradiation.
- Aim the source away from any living organism - to prevent irradiation.
- Store the source in a sealed container - to prevent contamination and irradiation.
Applications
| In a smoke detector alpha particles are released between two electrodes.
When the alpha particles ionise the molecules in the air the ions move between the electrodes and a current flows in the circuit. When smoke gets between the electrodes the ions become stuck in large particles of soot preventing them from moving. This stops the current in the circuit. The ammeter in the circuit is connected to an alarm which goes off when there is no current. |
Equation
\(_Z^AX \rightarrow _{Z-2}^{A-4}Y + _2^4\alpha\)
\(_{92}^{238}U \rightarrow _{90}^{234}Th + _2^4\alpha\)
Example Calculations
| Find the element 'X' and calculate its relative atomic mass 'A' and its relative atomic charge 'Z'.
\({}_{84}^{210}Po \rightarrow {}_{Z}^{A}X + {}_2^4\alpha\) |
Find the element 'X' and calculate its relative atomic mass 'A' and its relative atomic charge 'Z'.
\({}_{Z}^{A}X \rightarrow {}_{86}^{222}Rn + {}_2^4\alpha\) |
| 1. Calculate the relative atomic mass by looking at the top row of numbers.
210 = A + 4 A = 206 |
1. Calculate the relative atomic mass by looking at the top row of numbers.
A = 222 + 4 A = 226 |
| 2. Calculate the relative atomic charge by looking at the bottom row of numbers.
84 = Z + 2 Z = 82 |
2. Calculate the relative atomic charge by looking at the bottom row of numbers.
Z = 86 + 2 Z = 88 |
| 3. Since the relative atomic charge is the same as the atomic number look up the element on the periodic table.
\({}_{82}^{206}Pb\) |
3. Since the relative atomic charge is the same as the atomic number look up the element on the periodic table.
\({}_{88}^{226}Ra\) |
References
AQA
- Alpha decay, pages 109, 112-3, 116, GCSE Physics; Student Book, Collins, AQA'
- Alpha particle scattering experiment, pages 108, 109, GCSE Combined Science Trilogy; Physics, CGP, AQA'
- Alpha particle scattering experiment, pages 120, 121, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA'
- Alpha particle, page 21, GCSE Chemistry; Student Book, Collins, AQA'
- Alpha particle, pages 109, 112-6, 132-3, GCSE Physics; Student Book, Collins, AQA'
- Alpha particle; scattering experiment, pages 132-3, GCSE Physics; Student Book, Collins, AQA'
- Alpha particles, page 94, GCSE Physics, Hodder, AQA'
- Alpha particles, pages 114, 116, 122, GCSE Combined Science Trilogy; Physics, CGP, AQA'
- Alpha particles, pages 126, 128, 134, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA'
- Alpha particles, pages 344, 345, GCSE Combined Science Trilogy 1, Hodder, AQA'
- Alpha particles; effects on the body, page 102, GCSE Physics, Hodder, AQA'
- Alpha particles; properties of, pages 346, 347, GCSE Combined Science Trilogy 1, Hodder, AQA'
- Alpha particles; properties of, pages 96, 97, GCSE Physics, Hodder, AQA'
- Alpha radiation, pages 198, 199, GCSE Combined Science; The Revision Guide, CGP, AQA'
- Alpha radiation, pages 43-45, 47, GCSE Physics; The Revision Guide, CGP, AQA'
- Alpha radiation, pages 92-93, 96, 98-99, 109, GCSE Physics; Third Edition, Oxford University Press, AQA'
- Alpha radiation; dangers, page 201, GCSE Combined Science; The Revision Guide, CGP, AQA'
Edexcel
- Alpha decay, pages 156-159, GCSE Physics, CGP, Edexcel
- Alpha decay; dangers, pages 165, 166, GCSE Physics, CGP, Edexcel
- Alpha decay; uses, pages 168, 171, 172, GCSE Physics, CGP, Edexcel
- Alpha particles, page 362, GCSE Combined Science, Pearson Edexcel
- Alpha particles, page 98, GCSE Physics, Pearson Edexcel
- Alpha particles, pages 156, 157, GCSE Physics, CGP, Edexcel
- Alpha particles, pages 174, 175, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Alpha particles, pages 51, 52, GCSE Physics; The Revision Guide, CGP, Edexcel
- Alpha particles; scattering experiment, pages 149, 150, GCSE Physics, CGP, Edexcel
OCR
- Alpha particles, pages 20, 172-174, Gateway GCSE Physics, Oxford, OCR
- Alpha radiation, pages 197, 198, 200, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Alpha radiation, pages 74-76, 78, 79, Gateway GCSE Physics; The Revision Guide, CGP, OCR
Key Stage 5
Meaning
Alpha radiation consists of alpha particles emitted by certain radioactive materials, each composed of two protons and two neutrons.
About Alpha Radiation
- Alpha particles are helium nuclei.
- They can be stopped by a sheet of paper or human skin.
- Poses a health risk if alpha-emitting substances are inhaled or ingested.
- Alpha radiation has a high ionizing power, causing significant damage to living cells.
- Because of their high mass and charge, alpha particles have a short range in air (a few centimeters).
- Alpha radiation is used in radiation therapy for treating certain cancers.
- It is also used in smoke detectors and in some types of radioactive power sources (radioisotope thermoelectric generators).
Examples
- Polonium-210 emits alpha particles and is highly toxic if ingested.
- Alpha radiation is used in some types of smoke detectors.