Difference between pages "GCSE Physics Required Practical: Investigating Hooke's Law" and "GCSE Physics Required Practical: Investigating Thermal Insulators"
(Difference between pages) (→Improving Accuracy) |
|||
| Line 1: | Line 1: | ||
==Key Stage 4== | ==Key Stage 4== | ||
===Meaning=== | ===Meaning=== | ||
| − | Investigate the | + | Investigate the effectiveness of different [[Thermal Insulator|thermal insulators]] on reducing an [[Energy Transfer|energy transfer]] by [[heating]]. |
| − | + | ===Variables=== | |
| − | + | : [[Independent Variable]]: [[Thermal Insulator|Insulating material]]. | |
| − | : [[Independent Variable]]: | + | : [[Dependent Variable]]: The [[temperature]] decrease of [[water]] after a given [[time]]. |
| − | : [[Dependent Variable]]: The [[ | + | : [[Control Variable]]s: The [[Volume (Space)|volume]] of [[water]] used. The initial [[temperature]] of the [[water]]. The [[time]] taken between [[measurement]]s. Thickness of the [[Thermal Insulator|insulating material]]. |
| − | : [[Control Variable]]s: The [[ | ||
| − | + | ===Method=== | |
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| − | |[[File: | + | |[[File:RequiredPracticalThermalInsulation1.png|center|600px]] |
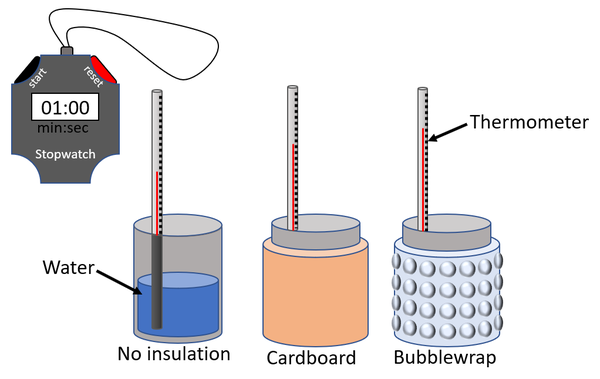
| − | | style="height:20px; width:200px; text-align:center;" |A [[diagram]] of the [[apparatus]] used | + | |- |
| + | | style="height:20px; width:200px; text-align:center;" |A [[diagram]] of the [[apparatus]] used in an [[experiment]] to compare the effectiveness of different [[Thermal Insulator|thermal insulators]]. | ||
|} | |} | ||
| − | + | #Fill a can with hot [[water]] (above 80°C). | |
| − | # | + | #Place a [[thermometer]] in the [[water]] and wait until the [[temperature]] reaches 80°C. |
| − | # | + | #Begin a [[stopwatch]] when the [[temperature]] is 80°C then [[Reading|read]] the [[temperature]] every 30 seconds for 5 minutes. |
| − | + | #Repeat steps 1-3 with a can covered each type of [[Thermal Insulator|insulating material]]. | |
| − | # | ||
| − | #Repeat steps | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | + | ===Improving [[Accuracy]]=== | |
| − | : | + | : Place the cans on a [[Heatproof Mat|heatproof mat]] to reduce the [[Thermal Energy Store|thermal energy]] lost to the table surface by [[Thermal Conduction|conduction]]. |
| − | : | + | : Place a lid over the cans to reduce the [[Thermal Energy Store|thermal energy]] lost to the [[air]] by [[evaporation]]. |
| + | : Complete the experiments with different [[Thermal Insulator|insulators]] simultaneously so that a change in the [[temperature]] of the [[laboratory]] does not affect the [[results]]. | ||
| + | ===Improving [[Precision]]=== | ||
| + | : Use a [[thermometer]] with a higher [[resolution]]. | ||
| + | : Use a [[Data Logger|data logger]] rather than a [[thermometer]]. | ||
Revision as of 19:05, 18 March 2019
Contents
Key Stage 4
Meaning
Investigate the effectiveness of different thermal insulators on reducing an energy transfer by heating.
Variables
- Independent Variable: Insulating material.
- Dependent Variable: The temperature decrease of water after a given time.
- Control Variables: The volume of water used. The initial temperature of the water. The time taken between measurements. Thickness of the insulating material.
Method
| A diagram of the apparatus used in an experiment to compare the effectiveness of different thermal insulators. |
- Fill a can with hot water (above 80°C).
- Place a thermometer in the water and wait until the temperature reaches 80°C.
- Begin a stopwatch when the temperature is 80°C then read the temperature every 30 seconds for 5 minutes.
- Repeat steps 1-3 with a can covered each type of insulating material.
Improving Accuracy
- Place the cans on a heatproof mat to reduce the thermal energy lost to the table surface by conduction.
- Place a lid over the cans to reduce the thermal energy lost to the air by evaporation.
- Complete the experiments with different insulators simultaneously so that a change in the temperature of the laboratory does not affect the results.
Improving Precision
- Use a thermometer with a higher resolution.
- Use a data logger rather than a thermometer.