Difference between revisions of "GCSE Chemistry Required Practical: Concentration and Rate of Reaction"
| Line 14: | Line 14: | ||
#Measure 10ml of 2[[Molarity|M]] [[Hydrochloric Acid]] using a [[Measuring Cylinder]]. | #Measure 10ml of 2[[Molarity|M]] [[Hydrochloric Acid]] using a [[Measuring Cylinder]]. | ||
#Add the 10ml of [[Hydrochloric Acid]] to the [[Conical Flask]] while starting a [[stopwatch]]. | #Add the 10ml of [[Hydrochloric Acid]] to the [[Conical Flask]] while starting a [[stopwatch]]. | ||
| − | #Observe the cross from above, through the [[solution]]. When the cross is no longer | + | #Observe the cross from above, through the [[solution]]. When the cross is no longer visible through the [[solution]] stop the [[stopwatch]] and record the [[time]] in [[second]]s. |
#Repeat the [[experiment]] with 20ml [[Sodium Thiosulphate]] and 30ml [[Water]], 30ml [[Sodium Thiosulphate]] and 20ml [[Water]], 40ml [[Sodium Thiosulphate]] and 10ml [[Water]] and 50ml [[Sodium Thiosulphate]]. | #Repeat the [[experiment]] with 20ml [[Sodium Thiosulphate]] and 30ml [[Water]], 30ml [[Sodium Thiosulphate]] and 20ml [[Water]], 40ml [[Sodium Thiosulphate]] and 10ml [[Water]] and 50ml [[Sodium Thiosulphate]]. | ||
#Repeat the same [[experiment]] for all [[ratio]]s of [[Sodium Thiosulphate]] and [[Water]] a further two times to calculate an [[Mean Average|average]]. | #Repeat the same [[experiment]] for all [[ratio]]s of [[Sodium Thiosulphate]] and [[Water]] a further two times to calculate an [[Mean Average|average]]. | ||
Revision as of 14:30, 3 April 2019
Contents
Key Stage 4
Meaning
Investigate how concentration affects the rate of reaction.
Method
Observation of Rate by Opacity
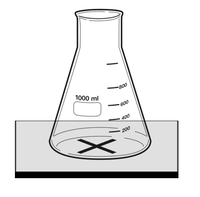
- Draw a large thick black cross on to a White Tile and place a 100ml Conical Flask on top as shown in the diagram.
- Measure 10ml of 0.25M Sodium Thiosulphate using a Measuring Cylinder and add to the Conical Flask.
- Measure 40ml of Water and add to the Conical Flask.
- Measure 10ml of 2M Hydrochloric Acid using a Measuring Cylinder.
- Add the 10ml of Hydrochloric Acid to the Conical Flask while starting a stopwatch.
- Observe the cross from above, through the solution. When the cross is no longer visible through the solution stop the stopwatch and record the time in seconds.
- Repeat the experiment with 20ml Sodium Thiosulphate and 30ml Water, 30ml Sodium Thiosulphate and 20ml Water, 40ml Sodium Thiosulphate and 10ml Water and 50ml Sodium Thiosulphate.
- Repeat the same experiment for all ratios of Sodium Thiosulphate and Water a further two times to calculate an average.
Observation of Rate by Gas Produced
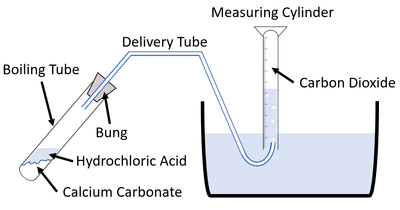
| This diagram shows a possible setup for measuring the gas given off during an experiment. |
- Set up the apparatus as shown in the diagram.
- Measure 10ml 2M Hydrochloric Acid using a Measuring Cylinder.
- Add the Hydrochloric Acid to the Boiling Tube.
- Add 1 spatula of Marble chips Calcium Carbonate to the Boiling Tube.
- Attach the bung.
- Fill the 50ml Measuring Cylinder with water in the tub and hold it over the end of the delivery tube to collect the bubbles.
- Start a stopwatch and record the volume of gas in the Measuring Cylinder every 10 seconds.
- Repeat this experiment with 10ml 1M Hydrochloric Acid.