Difference between revisions of "Litmus Paper"
| Line 17: | Line 17: | ||
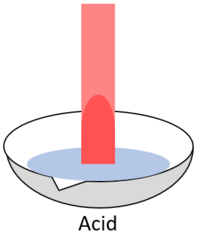
| style="height:20px; width:200px; text-align:center;" |When red '''litmus''' is placed in an [[acid]] it stays red. | | style="height:20px; width:200px; text-align:center;" |When red '''litmus''' is placed in an [[acid]] it stays red. | ||
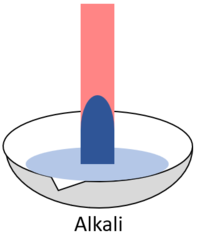
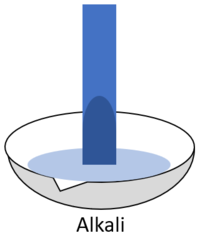
| style="height:20px; width:200px; text-align:center;" |When red '''litmus''' is placed in an [[alkali]] it turns blue. | | style="height:20px; width:200px; text-align:center;" |When red '''litmus''' is placed in an [[alkali]] it turns blue. | ||
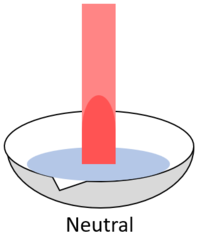
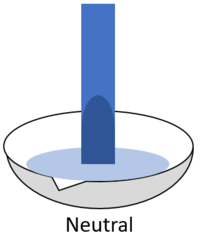
| − | | style="height:20px; width:200px; text-align:center;" |When red '''litmus''' is placed in a [[neutral]] [[solution]] it stays red. | + | | style="height:20px; width:200px; text-align:center;" |When red '''litmus''' is placed in a [[Neutral (Chemistry)|neutral]] [[solution]] it stays red. |
|- | |- | ||
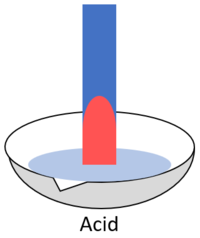
|[[File:BlueLitmusAcid.png|center|200px]] | |[[File:BlueLitmusAcid.png|center|200px]] | ||
Revision as of 16:34, 8 April 2019
Key Stage 3
Meaning
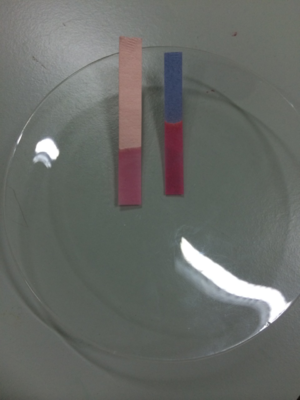
Two pieces of litmus paper dipped in an acid showing the red litmus stayed red but the blue litmus turned red.
Litmus paper is a piece of paper coloured with a dye that turns red in acid and blue in alkali.
About Litmus Paper
- Litmus paper comes in two colours: Red and Blue.
- Litmus paper is a very simple indicator as it can only tell if something is acid or alkali but it cannot tell the exact pH of a solution.
- Litmus paper cannot be used on a base unless it is in solution.
| When red litmus is placed in an acid it stays red. | When red litmus is placed in an alkali it turns blue. | When red litmus is placed in a neutral solution it stays red. |
| When blue litmus is placed in an acid it turns red. | When blue litmus is placed in an alkali it stays blue. | When blue litmus is placed in a neutral solution it stays blue. |