Difference between revisions of "Smelting"
| Line 10: | Line 10: | ||
|[[File:SmeltingFurnace.png|center|600px]] | |[[File:SmeltingFurnace.png|center|600px]] | ||
|- | |- | ||
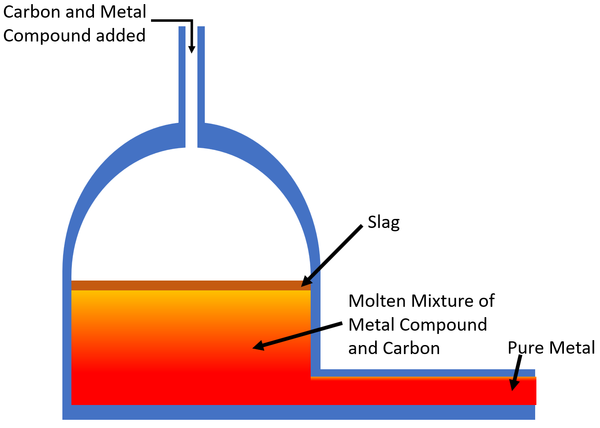
| − | | style="height:20px; width:200px; text-align:center;" |The [[Carbon]] [[Chemical Reaction|reacts]] with the [[mineral]] in a [[Displacement Reaction|displacement reaction]] creating usually producing [[Carbon Dioxide]] and the [[pure]] [[metal]] which is removed from the furnace. Any [[Impurity|impurities]] in the [[mineral]] rise to the top and make a layer called | + | | style="height:20px; width:200px; text-align:center;" |The [[Carbon]] [[Chemical Reaction|reacts]] with the [[mineral]] in a [[Displacement Reaction|displacement reaction]] creating usually producing [[Carbon Dioxide]] and the [[pure]] [[metal]] which is removed from the furnace. Any [[Impurity|impurities]] in the [[mineral]] rise to the top and make a layer called slag which is removed. |
|} | |} | ||
Revision as of 19:30, 8 April 2019
Contents
Key Stage 3
Meaning
Smelting is a process for extracting metals by heating and melting a mineral with carbon to cause a displacement reaction.
About Smelting
- Any metal below Carbon on the Reactivity Series can be extracted from its by smelting with Carbon.
- When the ore is melted it Carbon is added and a displacement reaction happens where the Carbon replaces the metal in the mineral.
| The Carbon reacts with the mineral in a displacement reaction creating usually producing Carbon Dioxide and the pure metal which is removed from the furnace. Any impurities in the mineral rise to the top and make a layer called slag which is removed. |
Example Reactions
- Iron Oxide + Carbon → Carbon Dioxide + Iron
- Zinc Oxide + Carbon → Carbon Dioxide + Zinc
- Tin Oxide + Carbon → Carbon Dioxide + Tin
- Lead Oxide + Carbon → Carbon Dioxide + Lead
Key Stage 4
Meaning
Smelting is a process for extracting metals by heating and melting a mineral with carbon to cause a displacement reaction.
About Smelting
- Any metal below Carbon on the Reactivity Series can be extracted from its by smelting with Carbon.
- When an ore containing a metal oxide is melted Carbon is added and a displacement reaction happens where the Carbon replaces the metal in the mineral.
- When an ore containing a metal carbonate a displacement reaction and a thermal decomposition reaction take place.
- Smelting requires a lot of energy to heat the mineral beyond its melting point so it can be expensive to carry out.
Example Reactions
Iron Oxide + Carbon → Carbon Dioxide + Iron
- <chem>2Fe2O3 + 3C -> 3CO2 + 4Fe</chem>
Zinc Oxide + Carbon → Carbon Dioxide + Zinc
- <chem>2ZnO + C -> CO2 + 2Zn</chem>
Tin Oxide + Carbon → Carbon Dioxide + Tin
- <chem>SnO2 + C -> CO2 + Sn</chem>
Lead Oxide + Carbon → Carbon Dioxide + Lead
- <Chem>2PbO + C -> CO2 + 2Pb</chem>
Zinc Carbonate + Carbon → Carbon Dioxide + Zinc
- <chem>2ZnCO3 + C -> 3CO2 + 2Zn</chem>
Copper Carbonate Hydroxide + Carbon → Carbon Dioxide + Water + Copper
- <chem>Cu2(CO3)(OH)2 + C -> 2CO2 + H2O + 2Cu</chem>